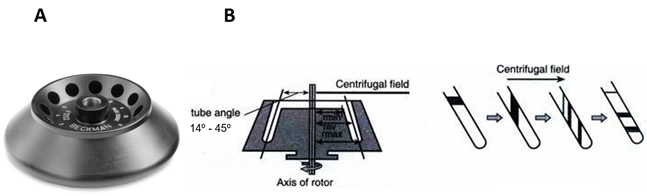
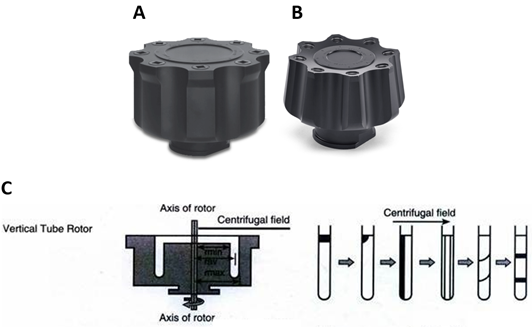
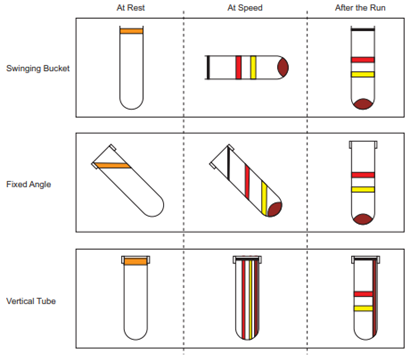
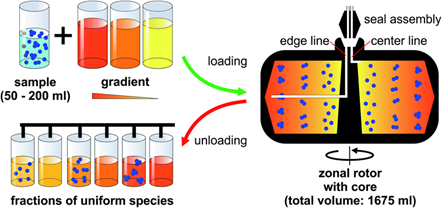
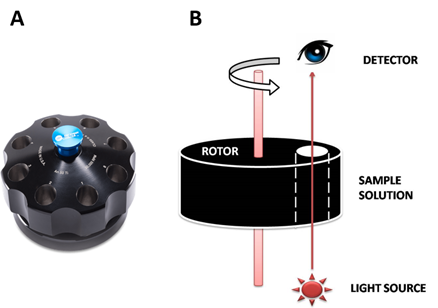
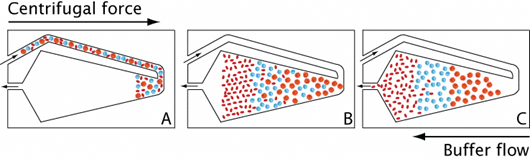
Adélia Mendes
Adélia Mendes is a science researcher in molecular biology. She is currently finishing her PhD about the effects of specific chromosomal translocations in the cellular proteome and their impact in intracellular transport in aggressive forms of leukemia. She graduated in Biochemistry at the University of Porto (Portugal) and has a master’s degree in molecular oncology also from the University of Porto (Portugal). Adélia moved to Brussels in 2015 to do her PhD, which is now in the finishing line. She has a passion for molecular oncology and translating the fundamental concepts of biology to real patients, where context can change all. In the course of her research experience, Adélia contributed as an author to several publications, from original reports, to systematic reviews and a book chapter on the effects of Leukemogenic nucleoporin fusion proteins and nucleocytoplasmic transport from Springer Book Series. Apart from doing and loving science research she loves cooking. She is a photography and digital editing enthusiast. However, her truly happy place is anywhere with a cup of coffee and a good book.