[vc_row type=”full_width_background” full_screen_row_position=”middle” column_margin=”default” bg_color=”#ffffff” scene_position=”center” text_color=”dark” text_align=”left” top_padding=”2%” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/4″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][/vc_column][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/2″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
About
[/vc_column_text][vc_column_text]The Koos Score is commonly used for patient self reported assessments of knee and associated problems. It has been validated in hundreds of studies across the world with multiple translations and widely used for research purposes, clinical trials, large scale databases, and registries. It also can be applied to multiple knee injury pathologist including ACL, meniscus injuries, chondral injuries, and has both long and short term interval assessments.[/vc_column_text][/vc_column][vc_column centered_text=”true” column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/4″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
Request
[/vc_column_text][contact-form-7 id=”102559″][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
Pricing
[/vc_column_text][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][pricing_table style=”default”][pricing_column color=”Accent-Color” title=”Monthly” id=”1579621991052-2″ tab_id=”1579621991052-4″ price=”5″ currency_symbol=”$” interval=”Per Patient/Month”]
- 3 Years data storage past end of collection date
- Unlimited sampling intervals during collection period.
[/pricing_column][pricing_column color=”Accent-Color” title=”1 Year” id=”1579621991178-10″ tab_id=”1579621991180-1″ price=”4.75″ currency_symbol=”$” interval=”Per Patient/Month”]
- 5% Discount
- 3 Years data storage past end of collection date
- Unlimited sampling intervals during collection period.
[/pricing_column][pricing_column color=”Accent-Color” title=”3 Years” id=”1579621991245-0″ tab_id=”1579621991247-4″ price=”4.00″ currency_symbol=”$” interval=”Per Patient/Month”]
- 20% Discount
- 3 Years data storage past end of collection date
- Unlimited sampling intervals during collection period.
[/pricing_column][/pricing_table][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
Documentation
[/vc_column_text][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
Introduction
[/vc_column_text][vc_column_text]This study explores the different uses and applications of Knee injury and Osteoarthritis Outcome Score (KOOS) research instrument. The development of KOOS was necessitated by the need of a single instrument which can be used in several types of knee injury and osteoarthritis (OA). Traumatic knee injuries can cause damage to multiple structures such as ligaments, menisci and cartilage among others. These injuries often lead to the later development of OA. Therefore, to be able to evaluate patients after a trauma and gain understanding in change of symptoms and function over time, a questionnaire which covers both the short-term and long-term consequences was needed.1
The KOOS was originally developed in 1995 by Ewa M. Roos and colleagues at the Departments of Orthopaedics at Lund University, Sweden and at the University of Vermont, USA.1 It is a self-administered knee-specific instrument, developed to assess the patient-reported knee outcomes in adults with joint injury or degenerative disease. It has been widely used for research purposes in clinical studies, databases and registries. The KOOS has been designed to evaluate both short-term and long-term consequences of knee injury. It holds 42 items in 5 separately scored subscales: pain, other symptoms, activities of daily living (ADL), sports and recreation (Sport/Rec), and knee-related quality of life (QOL).1.It is used in patients between 13-79years of age.2
Historically, the Knee injury and Osteoarthritis Outcome Score (KOOS) was also being used with children. But after cognitive interviews with 34 Swedish children between the ages of 10 and 16 years old were conducted, it was found that, children who had undergone knee surgery or physiotherapy had a greater understanding of the medical terminology than those who had not. Due to this lack of comprehension in those who were not familiar with the terminology or younger children, the KOOS had to be modified for them. Thus, a preliminary KOOS-Child version (LK 1.0), suitable for children aged between 9-12 years, was developed in 2012 before being updated to the current version, KOOS-Child (LK 2.0), in 2013.3
The KOOS-Child (LK. 2.0) also consists of 39 items divided in 5 subscales; Pain, other Symptoms (titled as “knee problems”), difficulty during daily activities (ADL), function in sport and play (Sport/Play) and knee related Quality of Life (QOL).3[/vc_column_text][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
Methods
[/vc_column_text][vc_column_text]The Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire is self-explanatory and can be administered in the waiting room or used as a mailed survey. The KOOS’s five important patient’s subscales are scored separately; Pain (9 items), other symptoms (7 items), function in daily living-ADL (17 items), function in sport and recreation-Sport/Rec (5 items), and knee related quality of Life-QOL (4 items). In the questionnaire, information on experience of the preceding week is considered. A Likert scale is used and all items have five possible answer options scored from 0 (No problems) to 4 (Extreme problems) and each of the five scores is calculated as the sum of the items included. For clinical interpretation, the five individual KOOS subscales scores are given as secondary outcomes. 2
To interpret scores, scores are transformed to a 0–100 scale, with zero representing extreme problems and 100 representing no knee problems as done in orthopaedic instruments and generic measures. Scores between 0 and 100 represent the percentage of total possible score achieved. The results of the five outcomes can be presented graphically as an outcome profile (order of subscales from left to right: Pain, Symptoms, ADL, Sport/Rec and QOL). This is in a graph with scores ranging from 0 -100 on the y-axis and the five outcomes on the x-axis.2[/vc_column_text][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
Applications
[/vc_column_text][vc_column_text]The KOOS has been validated for measuring outcomes in Total knee replacement (TKR), post-traumatic Knee osteoarthritis, 1 and anterior cruciate ligament (ACL) reconstruction2. The KOOS has also been used to evaluate other osteoarthritis interventions including minor knee surgery procedures, 4 conservative treatments, 5 nutritional 6 and pharmacological interventions. 7 Results from the studies show high response rates for studies of TKR in the short term, 92% at 6 months and 86% at 12 months. 8 A short-form version (KOOS-PS) which is a 7-item questionnaire derived from the original KOOS has been validated for evaluating physical function in individuals with knee OA undergoing TKR. 9[/vc_column_text][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
Comparisons
[/vc_column_text][vc_column_text]The KOOs questionnaire covers both the short-term and long-term consequences. Unlike prior instruments such as the Lysholm knee scoring scale which focused only on the short-term consequences, 10 and instruments such as the WOMAC Osteoarthritis Index which focused only on the long-term consequences. 11
The KOOS instrument is also highly reliable and responsive to change in subjects with knee injury.2 Compared to other knee outcome measures such as the the Tegner Activity Scale,10 the Lysholm Knee Scoring Scale,12 and International Knee Documentation Committee, Subjective Knee Form (IKDC), 13 which result in a single total score, the KOOS produces 5 subscores. It includes separate subscales for assessment of ADL function and higher physical function in sports and recreation, and also a scale for knee-related QoL, which are not present in other means of measuring knee outcome. 2
[/vc_column_text][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
Translations
[/vc_column_text][vc_column_text]The English and Swedish versions were developed concurrently. Numerous validated and translated versions are available in: Austria-German, Chinese, Croatian, Czech, Danish, Dutch, Estonian, French, German, Greek, Hindi (India), Italian, Japanese, Korean, Latvian, Lithuanian, Norwegian, Persian, Polish, Portuguese, Russian, Singapore English, Slovakian, Slovenian, Spanish (Peru), Spanish (US), Thai, Turkish, and Ukrainian.14 All the available language versions can be downloaded as pdf files from http://www.koos.nu/kooslanguage.html[/vc_column_text][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
Results and Sample Data Analysis
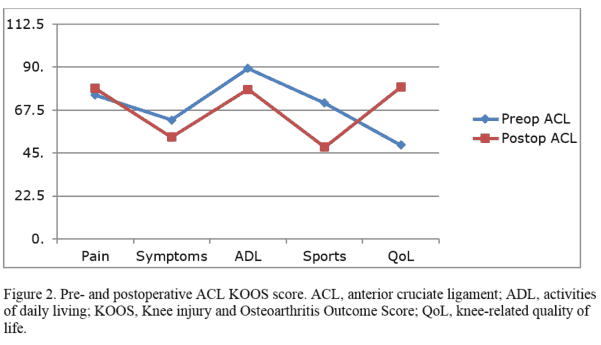
[/vc_column_text][vc_column_text]Example of results presentation and analysis of a study with the objective of evaluating postoperative results 2 years after primary PCL reconstruction and comparing the results to postoperative results 2 years after primary ACL reconstruction. Hypothesis of the study is; Patients with isolated posterior cruciate ligament (PCL) injuries improve from surgery as much as patients with anterior cruciate ligament ACL injuries after 2 years.
The patients complete the KOOS report preoperatively and at 2, 5, and 10 years postoperatively. Mean KOOS subscale scores for the different subgroups are calculated preoperatively for both the ACL and PCL groups. These values are then compared with the corresponding values at the 2-year follow-up, and 95% confidence intervals (CIs) are calculated based on paired-sample t tests. The changes in the PCL patients are compared with the relative changes in the control group (ACL patients). The chi-square test is used to compare the categorical data. The correlation is then calculated using the Pearson correlation coefficient[/vc_column_text][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
Scoring
[/vc_column_text][vc_column_text]The five dimensions of KOOS are scored separately: pain (nine items); symptoms (seven items); activities of daily life function (17 items); sport and recreation function (five items); and knee-related quality of life (four items). All items are scored from 0 to 4, and each of the five scores are calculated as the sum of the items included, in accordance with score calculations of the WOMAC Osteoarthritis Index (Table 1).1
 [/vc_column_text][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
[/vc_column_text][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
Strengths & Weaknesses
[/vc_column_text][vc_column_text]Strengths
The inclusion of two different subscales of physical function relating to daily life, and sport and recreation, enhances the KOOS’s validity for patients with a wide range of current and expected physical activity levels. It has also been translated and culturally adapted into many different languages. Since KOOS was predicated on the WOMAC survey and contains many of the same questions, a WOMAC score can be calculated from the KOOS questionnaire.2 In addition, the KOOS can be used for both short and long-term outcome reporting. This means it can be used to assess weekly changes that are induced by treatments or over years following a primary injury or osteoarthritis.2
Weaknesses
Compared to other questionnaires, the KOOS is longer and takes patients a considerable amount of time to fill out. It is also complex and can be laborious.[/vc_column_text][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
Summary and Key Points
[/vc_column_text][vc_column_text]
- The Knee injury and Osteoarthritis Outcome Score (KOOS) research tool, is a self-administered knee-specific instrument, developed to assess the patient-reported knee outcomes in adults with joint injury or degenerative disease.
- It was developed due to the need to evaluate patients after a trauma and gain understanding in change of symptoms and function over time.
- Due to its inadequacies in children, KOOS-Child version was developed.
- The KOOS questionnaire is self-explanatory and can be administered in the waiting room or used as a mailed survey. It has five subscales which are scored separately; Pain (9 items), other symptoms (7 items), function in daily living-ADL (17 items), function in sport and recreation-Sport/Rec (5 items), and knee related quality of Life-QOL (4 items).
- The KOOS has been validated for measuring outcomes in Total knee replacement (TKR), post-traumatic Knee osteoarthritis and anterior cruciate ligament (ACL) reconstruction.
- The KOOS questionnaire covers both the short-term and long-term consequences. It is also highly reliable, valid and responsive to change in subjects with knee injury.
- The KOOS does not require any license to use. It is also freely available online and has been translated and culturally adapted into many different languages.
[/vc_column_text][/vc_column][/vc_row][vc_row type=”in_container” full_screen_row_position=”middle” column_margin=”default” scene_position=”center” text_color=”dark” text_align=”left” bottom_padding=”30″ overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none” shape_type=””][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” overlay_strength=”0.3″ column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
References
[/vc_column_text][vc_column_text]
- Roos, EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 2003: 1:64.
- Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee injury and osteoarthritis outcome score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther 1998; 28(02): 88–96.
- Örtqvist M, Iversen MD, Janarv P-M, Brostrom EW, Roos EM. Psychometric properties of the Knee injury and Osteoarthritis Outcome Score for Children (KOOS-Child) in children with knee disorders. Submitted October 2013.
- Hare KB, Lohmander LS, Christensen R, Roos EM. Arthroscopic partial meniscectomy in middle-aged patients with mild or no knee osteoarthritis: a protocol for a doubleblind, randomized sham-controlled multi-centre trial. BMC Musculoskelet Disord 2013; 14: 7.
- Ghasemi GA, Golkar A, Marandi SM. Effects of hata yoga on knee osteoarthritis. Int J Prev Med 2013; 4(01): 133–138.
- Riecke BF, Christensen R, Christensen, P. Comparing two low-energy diets for the treatment of knee osteoarthritis symptoms in obese patients: a pragmatic randomized clinical trial. Osteoarthritis Cartilage 2010: 18(06): 746–754.
- Skou ST, Roos EM, Laursen MB. Efficacy of multimodal, systematic non-surgical treatment of knee osteoarthritis for patients not eligible for a total knee replacement: a study protocol of a randomised controlled trial. BMJ Open 2012; 2(6): ID e002168.
- Peer MA, Lane J. The knee injury and osteoarthritis outcome score (KOOS): a review of its psychometric properties in people undergoing total knee arthroplasty. J Orthop Sports Phys Ther 2013; 43(01): 20– 28.
- Davis AM, Perruccio AV, Canizares M. Comparative, validity and responsiveness of the HOOS-PS and KOOS-PS to the WOMAC physical function subscale in total joint replacement for osteoarthritis. Osteoarthritis Cartilage 2009; 17 (07): 843–847.
- Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop 1985: 43-49.
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988; 15:1833-1840.
- Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med; 1982;10: 150–154.
- Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, Richmond JC, Shelborne KD. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med 2001; 29: 600–613.
- Goncalves RS, Cabri J, Pinheiro JP, Ferreira L. Crosscultural adaptation and validation of the Portuguese version of the knee injury and osteoarthritis outcome score (KOOS). Osteoarthritis Cartilage 2009; 17(09): 1156–1162.
[/vc_column_text][/vc_column][/vc_row]

