
New Technique Discovered For Easy Autism Detection in Kids
A new study has discovered a new technique that ensures autism spectrum disorder in kids is accurately and quickly detected by doctors. Previously discovered
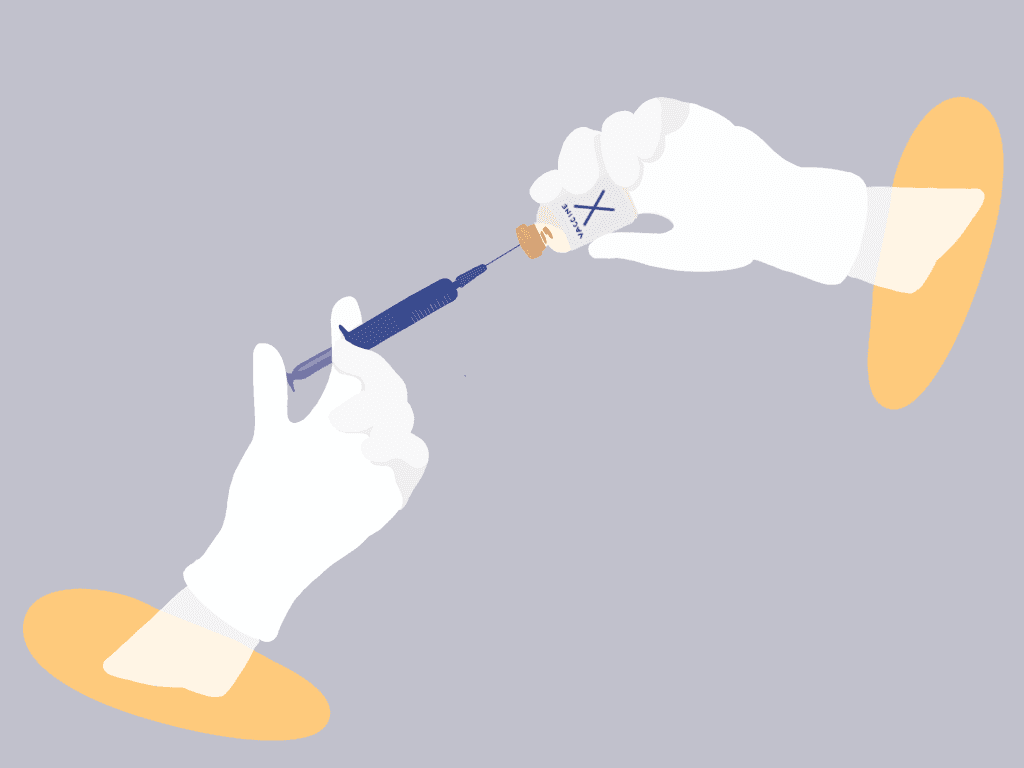
How vaccines work and the scientific innovation driving the process.
While vaccine research and development paradigms are well established and follow pertinent protocols, the COVID-19 crisis presents an opportunity to explore next-generation vaccine technology platforms and to consider safely and effectively accelerating the launch of a viable vaccine. As soon as the genetic sequence of the novel coronavirus that causes the disease, SARS-CoV-2, was published on January 11, 2020, the global science community began an enormous, coordinated effort to produce a solution. Now, as the world grapples with colossal humanitarian and economic consequences, it is clear that the path toward returning to normalcy is entirely contingent on the development of a successful vaccine. In broad terms, a vaccine is a substance that stimulates the body’s ability to mount an immune response and ultimately results in immunity to a disease. Currently, the World Health Organization reports that over 120 vaccine candidates have been proposed1. Before a successful vaccine can be delivered to the world’s population, however, each candidate must be rigorously assessed through a robust system of evaluation through clinical trials; it must be unequivocally clear and abundantly confident that the vaccine is effective and safe. As such, the process of vaccine development from start to finish can take years or even decades. Even so, the scientific community has already shown tremendous amounts of effort in order to address the urgent need for a COVID-19 vaccine. Of the candidates, thus far nearly 70 are in a pre-clinical evaluation stage with six already in clinical trial stages and another approved to begin1. There are several different methods, referred to as platforms, that deliver the key components that result in a vaccine’s prompted immune response and scientists are leaving no stone unturned in this regard. Of the seven clinical trial stage vaccine candidates, four are formulated with well-established platforms that serve as the basis for licensed vaccines, while three are based on next-generation vaccination technologies. One of these may likely be the solution that enables our return to normalcy—let’s break them down
This platform serves as the basis for two clinical-stage vaccine candidates, one developed by CanSino Biological Inc./Beijing Institute of Biotechnology (which was the first to begin clinical trials on March 17th) in China and one by the University of Oxford in England2. Both candidates stimulate the body’s immune response to SARS-CoV-2 by delivering the part of the genetic sequence disguised in the form of a defunct another form of virus (specifically an adenovirus). This approach exposes the immune system to the portion of the SARS-CoV-2 virus, the genetic sequence of the spike protein, that allows it to attach to human cells and therefore stimulates the production of antibodies3. The CanSino Biological Inc./Beijing Institute of Biotechnology vaccine has been reported to elicit a strong immune response in animal models and is now well underway in phase 2 of clinical trials which began on April 10th 4. Using the same method as their ongoing trial for another coronavirus, Middle East Respiratory Syndrome (MERS), the University of Oxford began phase 1/2 of the COVID-19 clinical trial on April 23rd 5.
This platform for vaccination has shown promise in animal studies for COVID-19 and is being used by two developers, the Beijing Institute of Biological Products/Wuhan Institute of Biological Products in China and Sinovac Biotech in China; both are in phase 1 of clinical trials starting April 10th and April 13th, respectively3. This vaccination method utilizes techniques to kill or inactivate a virus, grown in a controlled laboratory setting, with heat or chemicals so that the virus cannot replicate when administered to the body. Inactivated vaccines are commonly used to provide strengthened immunity to diseases, including influenza, pertussis, and polio, and as such are likely contenders for the current pandemic.
Although there are no licensed vaccines using this platform to date, the time and cost-efficient nature of this technology make DNA platforms of vaccination a rapidly developing field6. Under this approach, stimulation of immune response occurs by exposing the immune system to a key and recognizable component of a pathogen, a disease-causing agent, by tricking the cells into producing just that one identifiable piece. Exposure to SARS CoV-2 is simulated via DNA that codes for the SARS-CoV-2 spike protein7. Although DNA is enormously abundant in our cells, our body has enzymes that degrade DNA when it appears outside of the cell. In order to address this, Inovio Pharmaceuticals in the US is using novel technology, called electroporation, that allows the cells around the injection site to temporarily open and accept the vaccine to ensure enough of the DNA is able to stimulate the desired immune response. This vaccine began phase 1 of clinical testing on April 3rd with evidence that this vaccine stimulated immune response in mice and guinea pigs4.
RNA
The RNA-based vaccine is the most recent vaccine innovation to use nucleic acids, the molecules that carry information in cells, and have some advantages over DNA-based platforms7.
To date, there are no RNA vaccines approved for use, however, there are two COVID-19 and several other disease RNA vaccines in clinical trial stages. Moderna/National Institute for Allergies and Infectious Diseases in the US became the first RNA COVID-19 vaccine to start human trials, entering phase 1 on March 3rd, while BioNTech/Fosun Pharma/Pfizer has been approved to start the clinical testing of their RNA vaccine for the disease, trials have not yet begun2. RNA-based platforms for vaccination work in a very similar fashion as DNA-based platforms, but offer clear advantages; with no requirement of electroporation to ensure take up of the simulated virus exposure and a more easily constructed, smaller sequence, using RNA-based vaccines may well prove to be a solution to the current pandemic7.
While these are the most promising vaccines to date, the world will likely have to wait months to years with patience and trust the scientific process before there is complete return to the normal of the pre-COVID-19 times. Do not worry—science is on it.

A new study has discovered a new technique that ensures autism spectrum disorder in kids is accurately and quickly detected by doctors. Previously discovered

New Study shows that different coloration of primates doesn’t indicate fertile phases, but individual characteristics and reproductive status. Previous studies of ornamentation in female primates

Today astronomers from the Event Horizon Telescope Project (EHTP) unveiled the first-ever picture of a black hole and I’m sure most of you will have
A new study reveals that acute kidney failure can be detected earlier than the previous and currently used methods in patients. Drug-induced acute kidney




DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.