Zebrafish Mazes
Overview
The Zebrafish is a versatile animal model that is a common organism used in research. Much like the Drosophila animal model, Zebrafish are also popularly used in genetic screenings due to highly conserved genes and critical pathways between the species and humans. In comparison to traditional research animals, such as rats and mice, Zebrafish offer many technical advantages. The popularity of this model is due to its optical clarity and rapid embryonic development outside the mother which facilitates genetic studies. Zebrafish are hardy creatures that are easy to maintain in a laboratory set-up and are inexpensive.

-
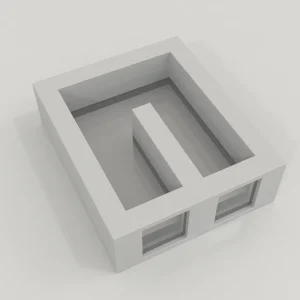
Zebrafish 3 Chamber Social Behavior
$1,990.00 Add to cart -
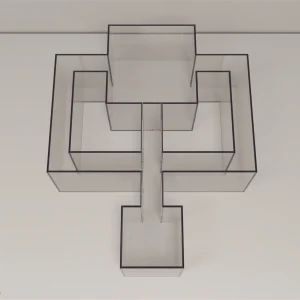
Latent Learning Apparatus
$1,990.00 Add to cart -
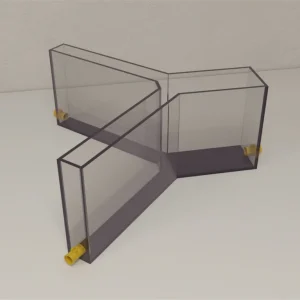
Automated Avoidance Zebrafish Y-Maze
$3,995.00 Add to cart -

Zebrafish Larvae Y Maze
$1,390.00 Add to cart -

Zebrafish Automated Home Tank
$1,690.00 Add to cart -
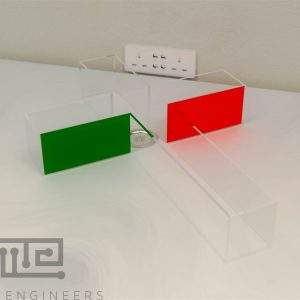
Zebrafish T Maze
$1,390.00 Select options This product has multiple variants. The options may be chosen on the product page
Zebrafish ConductVision: Enhancing Behavioral Research
ConductVision is an advanced behavioral tracking system designed for zebrafish studies. It enables researchers to analyze movement patterns, cognitive responses, and environmental interactions with high precision.
Key Features for Zebrafish Research:

Automated Tracking

Multi-Maze Compatibility

Data-Driven Insights

Light & Stimulus Detection

Zebrafish Behaviors and Characteristics
Scientific Classification
Scientific Name: | Danio rerio |
Family Name: | Cyprinidae |
Habitat: | Zebrafish live in freshwater streams and rivers. They are most often found in shallow; slow-moving water near the edge of streams or in ditches that have sandy substrates. |
Length: | In captivity Zebrafish rarely grow beyond 4 cm. In the wild; the fish can grow up to 6.4 cm in length. The fish are 1 cm wide. |
Diet: | Omnivorous |
Life Span: | 2 to 5 years in captivity under ideal conditions. |
Sexual Maturity: | 10 to 12 weeks |
Gestation Period: | Less than 24 hours |
Social Behavior: | Adult Zebrafish are robustly social animals exhibiting shoaling and schooling behaviors among other behaviors. |
Other behaviors & charasteristics
- Zebrafish have regenerative abilities
- Male Zebrafish have torpedo-shaped bodies with gold stripes between the blue stripes.
- Female Zebrafish have a larger, whitish belly and silver stripes instead of gold.
- Adult females exhibit a small genital papilla in front of the anal fin origin.
History
Zebrafish were first described by Francis Hamilton (Hamilton, Baird, & Swaine, 1822) as natives of the Indian floodplains living in diverse water habitats. Zebrafish were eventually introduced in Europe in the early 1900s as pet fishes and rose to popularity due to their beautiful diversity and ease of care. The introduction of species in the United States is believed to be the result of a deliberate release or by escape from fish farms.
In the 1930s, Charles Creaser began advocating the use of the fish in student laboratories and experimental research (Creaser, 1934). Though the 1940s to 1960s did see the use of Zebrafish in a range of research applications (Battle & Hisaoka, 1952; Hisaoka & Firlit, 1962; Weis, 1968), it wasn’t until the 1970s that the Zebrafish grew in fame. The most notable contribution to the popularity of the Zebrafish animal model was by American molecular biologist, George Streisinger. Streisinger, along with his colleagues at the University of Oregon, ventured out to find a vertebrate animal model that offered a better understanding in genetic research (Varga, 2018). One of Streisinger’s earliest research resulted in the creation of Zebrafish clones that were among the earliest successful vertebrate clones created (Streisinger, Walker, Dower, Knauber, & Singer, 1981). His work using Zebrafish (Chakrabarti, Streisinger, Singer, & Walker, 1983; Streisinger, 1984; Streisinger, Coale, Taggart, Walker, & Grunwald, 1989; Walker, & Streisinger, 1983) laid the foundation for Zebrafish animal model and earned him the title of founding father of Zebrafish Developmental and Genetic Research.
The success of Zebrafish animal model received another push when Christiane Nüsslein‐Volhard (Haffter et al., 1996) and Wolfgang Driever (Driever et al., 1996) performed genetic screens of Zebrafish with the aim to find pathways with deeper homology to key pathways in human development. Labeled “Big Screens,” Nüsslein‐Volhard’s screening involved the use of the chemical mutagen ethylnitrosourea (ENU) to mutagenize male sperm. The resulting mutant Zebrafish included mutations at approximately six hundred independent genetic loci. A special issue of the Development published the categorization and descriptions of 1163 mutant lines from the Big Screen in their 1996 journal publication. The Big Screen eventually aided the completion of genome sequencing of the Zebrafish in 2013 (Howe et al., 2013).
Training Considerations and Best Practices
As with any animal model, Zebrafish also require specific care and maintenance to ensure their well-being and extract their full potential for research. Zebrafish are hardy animals that are relatively easy to rear and maintain in a laboratory facility. The following are a few considerations and practices for using Zebrafish for research purposes. (To know more about zebrafish testing click here.)
- The ideal temperature for Zebrafish is between 26 °C and 28.5 °C with a pH between 6.8 and 7.5. The pH can be balanced by the addition of sodium bicarbonate.
- Zebrafish can be sensitive to chlorine and other chemicals. Therefore, the tank water should be dechlorinated either by aeration or a dechlorinating water conditioner.
- Tank water should be regularly changed and filtered to maintain water quality. Water lost due to evaporation should be replenished.
- It is recommended that the Zebrafish be habituated to the facility before being tested to avoid the stress of novelty. Additionally, stressors should be minimized as much as possible to prevent their influence on the subject’s behaviors.
- Zebrafish are social animals; hence they should be maintained in shoals to avoid isolation stress.
- Overfeeding of Zebrafish could result in increased levels of nitrate in the water and potentially affect the breeding and viability of the fishes.
- Breeding of the fishes should be undertaken regularly to ensure the breeding cycle of the fish is maintained. The optimal breeding conditions for Zebrafish is between 3 and 18 months.
- Feeding of larvae should begin from day 5 post-fertilization on dry diets and/or live foods such as paramecia or rotifers.
Zebrafish Behavioral Testing Equipment for Sale
At ConductVision, we offer a range of behavioral testing solutions tailored for zebrafish research laboratories
Visual Water Maze
Evaluate spatial learning and memory using visual cues to assess cognitive function in zebrafish.

Y Maze Zebrafish
Measure exploration, decision-making, and working memory by analyzing spontaneous alternation behavior.
Swim Tunnel Zebrafish
Assess swimming performance, endurance, and metabolic response under controlled water flow conditions.
Five Choice Zebrafish
Conduct attention-based and learning tasks, often used for cognitive and drug response studies.
Zebrafish in Research
Zebrafish is one of the most sought-after animal models to study gene function along with the Drosophila melanogaster. The ability to create gene knockdown or overexpression in addition to the almost transparent embryos have allowed acceleration of genetic studies. About 70% of protein-coding human genes and 84% of known genes associated with human diseases have been found to have homologs in the Zebrafish (Howe et al., 2013; Kettleborough et al., 2013). Zebrafish cover a range of research domains including regeneration studies, drug testing and screening, and cancer research.
Strengths and Limitations
References
Ali, S., Champagne, D. L., Spaink, H. P., & Richardson, M. K. (2011). Zebrafish embryos and larvae: a new generation of disease models and drug screens. Birth Defects Research. Part C, Embryo Today. 2011 Jun;93(2):115-33. doi: 10.1002/bdrc.20206.
Audira, G., Sarasamma, S., Chen, J.-R., Juniardi, S., Sampurna, B., Liang, S.-T., … Hsiao, C.-D. (2018). Zebrafish Mutants Carrying Leptin a (lepa) Gene Deficiency Display Obesity, Anxiety, Less Aggression and Fear, and Circadian Rhythm and Color Preference Dysregulation. International Journal of Molecular Sciences, 19(12), 4038. doi:10.3390/ijms19124038
Avdesh, A., Chen, M., Martin-Iverson, M. T., Mondal, A., Ong, D., Rainey-Smith, S., …, Martins RN. (2012). Regular care and maintenance of a zebrafish (Danio rerio) laboratory: an introduction. Journal of Visual Experiments, (69):e4196. doi: 10.3791/4196.
Battle, H. I., & Hisaoka, K. K. (1952). Effects of ethyl carbamate (urethan) on the early development of the teleost Brachydanio rerio. Cancer Research,12(5):334-40.
Bui-Klimke, T. R., & Wu, F. (2015). Ochratoxin A and human health risk: a review of the evidence. Critical Reviews in Food Science and Nutrition, 55(13):1860-9. doi: 10.1080/10408398.2012.724480.
Chakrabarti, S., Streisinger, G., Singer, F., & Walker, C. (1983). Frequency of gamma-Ray Induced Specific Locus and Recessive Lethal Mutations in Mature Germ Cells of the Zebrafish, BRACHYDANIO RERIO. Genetics, 103(1):109-23.
Creaser, C. W. (1934). The Technic of Handling the Zebra Fish (Brachydanio rerio) for the Production of Eggs Which Are Favorable for Embryological Research and Are Available at Any Specified Time Throughout the Year. Copeia,1934(4), 159. doi:10.2307/1435845
Driever, W., Solnica-Krezel, L., Schier, A. F., Neuhauss, S. C., Malicki, J., Stemple, D. L., …, Boggs C. (1996). A genetic screen for mutations
affecting embryogenesis in zebrafish.
Development,123:37-46.
Echevarria, D. J., Caramillo, E. M., & Gonzalez-Lima, F. (2016). Methylene Blue Facilitates Memory Retention in Zebrafish in a Dose-Dependent Manner. Zebrafish, 13(6):489-494.
Faillace, M. P., Pisera-Fuster, A., & Bernabeu, R. (2018). Evaluation of the rewarding properties of nicotine and caffeine by implementation of a five-choice conditioned place preference task in zebrafish. Progress in Neuropsychopharmacology and Biological Psychiatry, 84(Pt A):160-172. doi: 10.1016/j.pnpbp.2018.02.001.
Haffter, P., Odenthal, J., Mullins, M. C., Lin, S., Farrell, M. J., Vogelsang, E., …, Nüsslein-Volhard C. (1996). Mutations affecting pigmentation and shape of the adult zebrafish. Development Genes and Evolution, 206(4):260-76. doi: 10.1007/s004270050051.
Hamilton, F., Baird, S. F., & Swaine, J. (1822). An account of the fishes found in the river Ganges and its branches. doi:10.5962/bhl.title.59540
Hisaoka, K. K., & Firlit, C. F. (1962). The localization of nucleic acids during oogenesis in the zebrafish. The American Journal of Anatomy, 110:203-15.
Howe, H. B., McIntyre, P. B., & Wolman, M. A. (2018). Adult zebrafish primarily use vision to guide piscivorous foraging behavior. Behavioural Processes. doi:10.1016/j.beproc.2018.10.005
Howe, K., Clark, M. D., Torroja, C. F., Torrance, J., Berthelot, C., Muffato, M., …, Stemple, D. L. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature,496(7446):498-503. doi: 10.1038/nature12111.
Jia, L., Raghupathy, R. K., Albalawi, A., Zhao, Z., Reilly, J., Xiao, Q., & Shu, X. (2017). A colour preference technique to evaluate acrylamide-induced toxicity in zebrafish. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 199, 11–19. doi:10.1016/j.cbpc.2017.01.004
Kettleborough, R. N., Busch-Nentwich, E. M., Harvey, S. A., Dooley, C. M., de Bruijn, E., van Eeden, F., …, Stemple, D. L. (2013). A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature, 496(7446):494-7. doi: 10.1038/nature11992
Kim, J., Oh, H., Ryu, B., Kim, U., Lee, J. M., Jung, C. R., …, Park, J. H. (2018). Triclosan affects axon formation in the neural development stages of zebrafish embryos (Danio rerio). Environmental Pollution, 236:304-312. doi: 10.1016/j.envpol.2017.12.110.
Liu, W., Zhang, X., Wei, P., Tian, H., Wang, W., & Ru, S. (2017). Long-term exposure to bisphenol S damages the visual system and reduces the tracking capability of male zebrafish (Danio rerio). Journal of Applied Toxicology, 38(2), 248–258. doi:10.1002/jat.3519
Marcon, M., Mocelin, R., Benvenutti, R., Costa, T., Herrmann, A. P., de Oliveira, D. L., … Piato, A. (2018). Environmental enrichment modulates the response to chronic stress in zebrafish. The Journal of Experimental Biology, 221(4), jeb176735. doi:10.1242/jeb.176735
Meyers, J. R. (2018). Zebrafish: Development of a Vertebrate Model Organism. Current Protocols Essential Laboratory Techniques, 16(1). doi:10.1002/cpet.19.
Naderi, M., Salahinejad, A., Ferrari, M. C. O., Niyogi, S., & Chivers, D. P. (2018). Dopaminergic dysregulation and impaired associative learning behavior in zebrafish during chronic dietary exposure to selenium. Environmental Pollution, 237:174-185. doi: 10.1016/j.envpol.2018.02.033.
Naderi, M., Wong, M. Y., & Gholami, F. (2014). Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquatic Toxicology, 148:195-203. doi: 10.1016/j.aquatox.2014.01.009.
Oliveri, A. N., & Levin, E. D. (2018). Dopamine D1 and D2 Receptor Antagonism During Development Alters Later Behavior in Zebrafish. Behavioural Brain Research. doi:10.1016/j.bbr.2018.08.028.
Parker, M. O., Annan, L. V., Kanellopoulos, A. H., Brock, A. J., Combe, F. J., Baiamonte, M., …, Brennan, C. H. (2014). The utility of zebrafish to study the mechanisms by which ethanol affects social behavior and anxiety during early brain development. Progress in Neuropsychopharmacology and Biological Psychiatry, 55:94-100. doi: 10.1016/j.pnpbp.2014.03.011.
Sarasamma, S., Varikkodan, M. M., Liang, S.-T., Lin, Y.-C., Wang, W.-P., & Hsiao, C.-D. (2017). Zebrafish: A Premier Vertebrate Model for Biomedical Research in Indian Scenario. Zebrafish, 14(6), 589–605. doi:10.1089/zeb.2017.1447
Saszik, S. M., & Smith, C. M. (2018). The impact of stress on social behavior in adult zebrafish (Danio rerio). Behavioural Pharmacology, 29(1), 53–59. doi:10.1097/fbp.0000000000000338.
Shams, S., Amlani, S., Buske, C., Chatterjee, D., & Gerlai, R. (2017). Developmental social isolation affects adult behavior, social interaction, and dopamine metabolite levels in zebrafish. Developmental Psychobiology, 60(1), 43–56. doi:10.1002/dev.21581.
Sheng, L., Wang, L., Su, M., Zhao, X., Hu, R., Yu, X., …, Hong, F. (2016). Mechanism of TiO2 nanoparticle-induced neurotoxicity in zebrafish (Danio rerio). Environmental Toxicology, 31(2):163-75. doi: 10.1002/tox.22031.
Streisinger, G. (1984). Attainment of minimal biological variability and measurements of genotoxicity: production of homozygous diploid
zebra fish. National Cancer Institute Monograph, 65:53-8.
Streisinger, G., Coale, F., Taggart, C., Walker, C., & Grunwald, D. J. (1989). Clonal origins of cells in the pigmented retina of the zebrafish eye. Developmental Biology, 131(1):60-9.
Streisinger, G., Walker, C., Dower, N., Knauber, D., & Singer, F. (1981). Production of clones of homozygous
diploid zebra fish (Brachydanio rerio). Nature, 291(5813):293-6.
Tavares, B., & Santos Lopes, S. (2013). The importance of Zebrafish in
biomedical research. Acta Medica Portuguesa, 26(5):583-92.
Varga, M. (2018). The Doctor of Delayed Publications: The Remarkable Life of George Streisinger (1927-1984). Zebrafish, 15(3):314-319. doi: 10.1089/zeb.2017.1531.
Volgin, A. D., Yakovlev, O. A., Demin, K. A., Alekseeva, P. A., & Kalueff, A. V. (2018). Acute behavioral effects of deliriant hallucinogens atropine and scopolamine in adult zebrafish. Behavioral Brain Research, 359:274-280. doi: 10.1016/j.bbr.2018.10.033.
Walker, C., & Streisinger, G. (1983). Induction of Mutations by gamma-Rays in Pregonial Germ Cells of Zebrafish Embryos.
Genetics, 103(1):125-36.
Weis, J. S. (1968). Analysis of the development of nervous system of the zebrafish, Brachydanio rerio. I. The normal morphology and development of the spinal cord and ganglia of the zebrafish. Journal of Embryology and Experimental Morphology, 19(2):109-19.
Wu, T. S., Lin, Y. T., Huang, Y. T., Cheng, Y. C., Yu, F. Y., & Liu, B. H. (2018). Disruption of liver development and coagulation pathway by ochratoxin A in embryonic zebrafish. Toxicology and Applied Pharmacology, 340:1-8. doi: 10.1016/j.taap.2017.12.012.
Zahid, H., Tsang, B., Ahmed, H., Lee, R. C. Y., Tran, S., & Gerlai, R. (2018). Diazepam fails to alter anxiety-like responses but affects motor function in a white-black test paradigm in larval zebrafish (Danio rerio). Progress in Neuro-Psychopharmacology and Biological Psychiatry, 83, 127–136. doi:10.1016/j.pnpbp.2018.01.012

