

Stereotaxic surgery, also known as stereotactic surgery, is a minimally invasive surgical technique used to precisely target areas within the brain. It is commonly employed in neurological research, the treatment of neurological disorders, and various types of biopsies.
The technique was first developed by Horsley and Clarke in the early 1900s for use in non-human primates. This method allows for the precise placement of brain implants, initially ablation electrodes, by utilizing a coordinate system applied to the brain. It wasn’t until 1947 that Spiegel and Wycis adapted this technique and device for use in humans [1].
It is extensively utilized in both preclinical and clinical research. In preclinical animal studies, this technique allows researchers to inject fluids into the brain and stimulate specific brain regions. Clinically, stereotactic neurosurgery is used to treat conditions such as Parkinson’s disease. For example, in a procedure called Pallidotomy, a small electrical probe is inserted into the patient’s globus pallidus, where it is heated to destroy surrounding brain cells and alleviate symptoms [2].
Today, stereotaxy is still widely used, particularly in laboratory animals such as rodents.
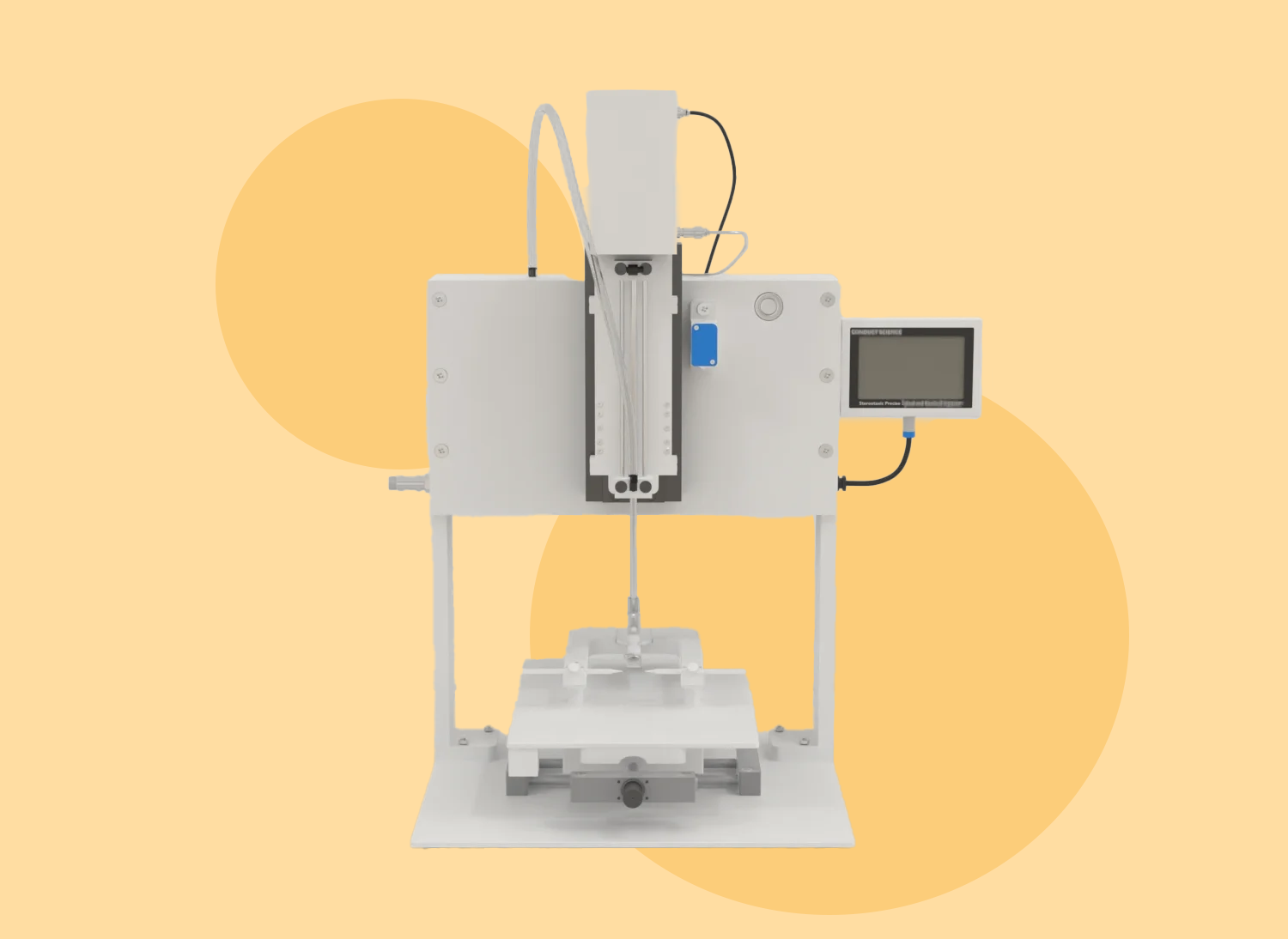
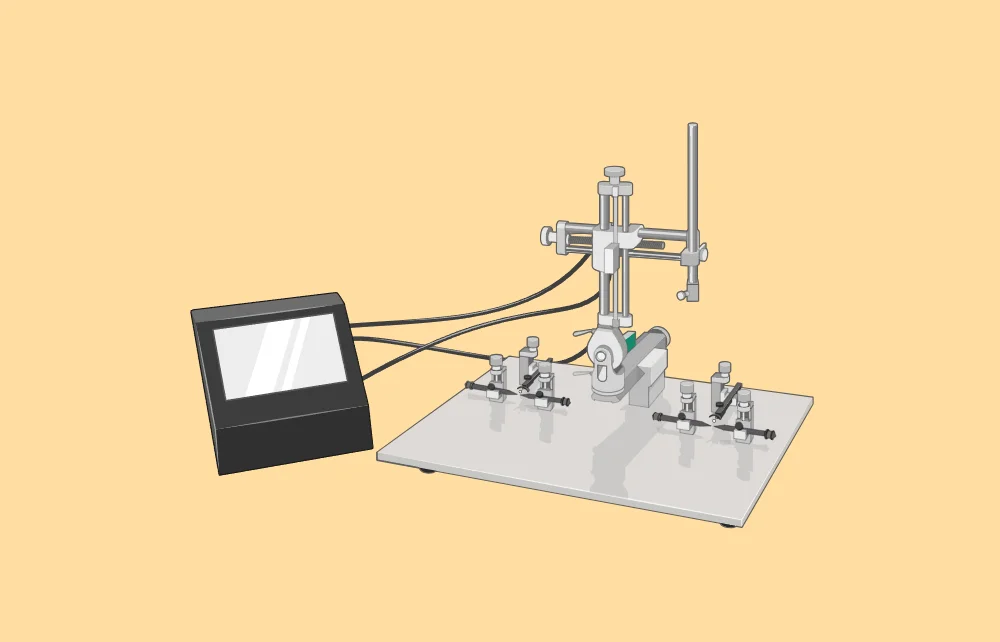
The stereotaxic apparatus is built around a U-shaped frame mounted on a base plate. This frame supports three mechanical elements that enable precise positioning of an electrode or cannula holder along three orthogonal axes: front-to-back, up-and-down, and side-to-side. The placement of the holder is adjusted using three micrometer Vernier screw drives for fine control.
The apparatus also includes two laterally adjustable ear bars and a height-adjustable incisor bar, which secure the animal’s head in a fixed position. The ear bars align the head parallel to the anteroposterior axis of the frame, while the incisor bar sets the head at the “flat-skull position.” This alignment ensures that the two reference points on the animal’s skull, the bregma and lambda, are on the same horizontal plane, facilitated by the incisor bar.
Precisely targeting hard-to-reach brain regions remains challenging, especially when dealing with midline brain structures. These challenges involve avoiding critical areas like the superior sagittal sinus and the third ventricle, and consistently targeting specific, discrete brain nuclei. Additionally, advanced neuroscience methods such as optogenetics, fiber photometry, and two-photon imaging require the precise implantation of significant hardware into the brain, with spatial constraints often posing a common obstacle. Study from 2020 (Faber et. al), presented a modifiable protocol for stereotactic targeting of rodent brain structures using an angled coronal approach that may be adapted for 1) either mouse or rat; 2) various neuroscience techniques and 3) for multiple brain regions [5]. You can find a comprehensive description of the protocol in the online article.
To ensure the longevity and precision of stereotaxic instruments, proper handling and storage are essential. After each surgical procedure, the stereotaxic apparatus and reusable instruments must be cleaned, decontaminated, and sterilized. Initially, wash the instruments with soap and water, thoroughly dry them, and then sterilize them using an autoclave. For more delicate materials such as cannulae, obturators, and electrodes, carefully decontaminate or autoclave them, considering their specific tolerances.
When cleaning the stereotaxic apparatus, use cotton swabs or paper towels soaked in a 70% ethyl alcohol antiseptic solution to clean the frame, ear bars, nose bars, tooth bars, verniers, heating pad, rectal temperature probe, and workbench.
Regular preventive maintenance and servicing of the equipment, based on usage frequency, are crucial for storage. These practices help maintain the instrument’s precision and prevent potential stereotaxic errors caused by loose or unstable components.



Dr Louise Corscadden acts as Conduct Science’s Director of Science and Development and Academic Technology Transfer. Her background is in genetics, microbiology, neuroscience, and climate chemistry.
