The animal is considered ready to be placed on the stereotaxic apparatus once the proper depth of surgical anesthesia is reached, as indicated by regular breathing and a characteristic loss of reflexes.
Placement of the Ear Bars, Tooth/Incisor Bar, and Nose Bar
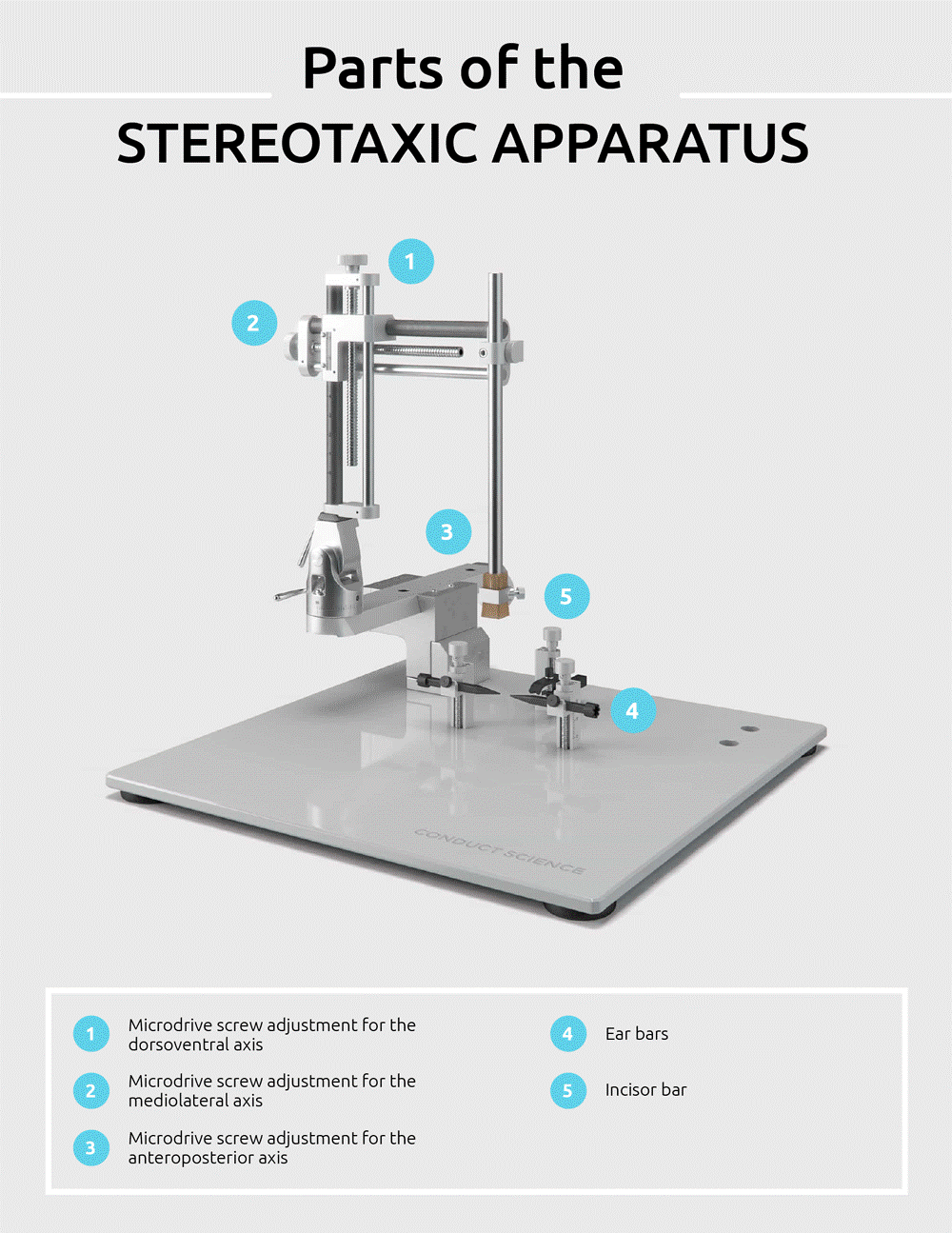
In securing the animal onto the set-up, one of the two ear bars must first be fixed in the instrument. Then, the head of the animal is gently steered so that the ear canal is guided towards the fixed ear bar. The head is then horizontally maneuvered so that the tip of the bar is positioned behind the aural bone spur. Once the bar is in place, a soft click will be heard. One must be careful to distinguish the sound from a louder snap, characteristic of a possible ruptured eardrum if the bar is inserted too far. Then, the second ear bar is guided into the other ear canal. Once both ear bars are properly placed, the head of the animal will only be able to shift in an Antero- and retroflexed fashion.
Once the ear bars are properly in place, the subject’s head may then be aligned with the center of the stereotaxic frame. The mouth of the animal is opened, and the movable tooth bar is slid behind its upper incisors. The head of the animal is then gently pulled forward so that the tooth bar may slide and click into its correct place.
The final step is locking the snout in place with the usage of the nose bar. The head is immobilized by fixing the nose bar’s setscrew at the proper setting, providing sufficiently firm and stable pressure on the snout. In this manner, no head movement shall be possible during the surgical procedure.
Preparation of the Operation Zone and Draping
After securing the animal in place, the skin on which the incision will be made is then disinfected by washing with sterile gauze soaked with antiseptic soap, alcohol, and an antiseptic solution. The standard washing procedure involves centrifugal strokes, away from the incision site towards the periphery. The gauze must not touch the edge of the shaved zone. The surgical drapes are used for the maintenance of sterility after the operation zone is cleaned. The drape should cover the entire animal, and can even extend to the adjacent areas, in order to widen the surface of the sterile zone.
Incision and Exposure of the Cranial Surface
A scalpel fitted with a new blade is used to incise the scalp. Precise scalpel handling is necessary, involving all layers of skin. The linear incision must start slightly in front of eye level and continue backward or caudally to approximately 0.5 cm behind the ear bars.
Stereotaxic Landmarks
Once the incision is made, the suture lines of the four bone plates of the skull surface must be visibly identified in order to locate the two reference points correctly; the bregma and lambda. The bregma pertains to the intersection of the sagittal and frontal sutures at the level of the snout, whereas the lambda, named for its resemblance to the Greek letter lambda, is a point located on the midline and at the base of the triangle formed by the lambdoid and sagittal sutures. Once the stereotaxic landmarks have been identified, the operator may carry on with the procedure, beginning with taking the coordinates of the two reference points using the tip of the instrument.
Applications
Stereotaxic procedures have been around for a long time, and up until now have much to contribute to the methodological principles of research in the neurosciences. The importance of knowledge acquired through stereotaxic surgery on rodents and other experimental animals is of considerable magnitude, given the successful extrapolation of findings to research on human biology and behavior. Beyond furthering knowledge, the impact of stereotaxy extends to the symptomatic treatment of motor disorders in humans such as Parkinson’s disease, as well as biopsies of tumoral pathologies and interstitial radiotherapies, and the treatment of other illnesses like epilepsy. Different techniques are used for different research objectives, and these include experimental procedures in vivo for the activation or inactivation of certain brain regions or transmitter systems, permanent selective lesioning, functional neuroanatomy through the use of tracers, and acute or chronic measurements or recordings (Ferry et al., 2014).
Inducing Lesions
One application of stereotaxic procedures on experimental animals lies in the investigation of behavioral functions mediated by different brain regions, by implementing an ablation of function. Procedures such as these involve experimentally inducing lesions of particular brain regions and studying the resulting deficits in function. There are plenty of ways to damage or deplete a brain structure or transmitter system through stereotaxic neurosurgery, such as electrolysis, transection, aspiration, radio frequency, injection of excitotoxins, or by global or focal ischemia through the use of devascularization, photo-irradiation, vessel occlusion, or vasoconstrictive agents. Additionally, stereotaxic neurosurgery can also be used for the temporary inactivation of a brain structure, through microinjection of muscimol, tetrodotoxin, lidocaine, or receptor antagonists at the target system.
Lesioning via electrolysis was first accomplished by Duncan et al., 1975 in which dopamine-containing nerve terminals were unilaterally destroyed through the use of unipolar electrolytic lesions of the medial forebrain bundle. Transection, meanwhile, is another reliable lesioning method that basically involves cutting across target brain structures. Milner and Amaral (1984) successfully created transections across projections of the septal complex to the hippocampus, in the attempt to investigate the existence of a ventral septohippocampal pathway. Moreover, some researchers argue that excitotoxic lesioning remains the most reliable and efficient method that guards against damage to local glial cells or traversing nerve fibers (Kirby et al., 2012). Extensive loss of brain cells surrounding target brain structures can conveniently be avoided by careful practice of excitotoxic focal injection (Jarrard, 2002). Researchers from UCLA and MIT (Kirby et al., 2012) also successfully developed a stereotaxic method allowing for excitotoxic lesions of specific brain areas through an infusion of N-methyl-D-aspartate (NMDA). Specifically, the infusion of NMDA into the brain results in the excitotoxic death of nearby neurons. The established protocol can also be used to infuse other biological compounds, such as viral vectors, and the implantation of more permanent osmotic pumps for prolonged exposure.
Furthermore, stereotaxic surgery also allows for the stimulation of certain brain regions through a number of techniques. These include electrical stimulation through a nonselective activation of a heterogeneous population of neurons, stimulation of NMDA-receptor-bearing neurons through microinjection of NMDA, and laser application succeeding transfection of modified gene sequences (Buchen, 2010). Through a combination of genetics, virology, and optics, optogenetics has provided a tool with which researchers can quickly activate or inactivate specific groups of neurons within particular neural circuits in a highly precise manner that is unachievable through other more traditional methods (Buchen, 2010).
Tracing of Neural Pathways
Retrograde and anterograde tracing, which involve the infusion of such substances as wheat germ agglutinin-conjugated horseradish peroxidase or Fast blue (Milner and Amaral, 1984), enable the tracing of neural pathways and particular brain structures as necessitated by some stereotaxic procedures.
Sampling Techniques
In addition, stereotaxy also allows for sampling techniques in rodents, such as microdialysis. In vivo measurements (measurements in a living organism) can be done through the concentration of extracellular neuromediators by microdialysis, voltammetry (Li et al., 2006), and electro potentials. The process of microdialysis provides quantification of various substances in blood and tissue, including neurotransmitters and neuropeptides, enzyme activity, electrolytes, and hormones as well as the monitoring of biochemical and physiological effects of pharmaceutical agents. Furthermore, the method also allows for more in-depth observation of drug effects on extracellular levels of endogenous substances (Li et al., 2006). Aside from its sampling function, microdialysis also permits the infusion of certain substances into the brain and spinal cord. The stereotaxic procedure would involve the insertion of a microdialysis probe with inlet and outlet tubes for perfusion and sample collection into the fluid or tissue compartment (Zapata et al., 2009).
Rodent Models of Human Diseases
Another extremely useful application of stereotaxic lesioning is the elaboration of rodent models of human diseases. For instance, a model involving platelet aggregation with regard to cerebral ischemia was developed by Watson et al., 1985 as an attempt to investigate the widespread incidence of human stroke cases due to vascular thrombosis. Jumping off on well-established findings on stroke cases, Bergeron (2003) successfully yielded a photothrombotic cortical stroke model by inducing photochemical cortical lesions in the rat brain using stereotaxic surgical procedures. Particularly, a minimally invasive protocol for creating a model of permanent focal ischemia was developed through the use of photochemical cortical lesions.
Stem-Cell Transplantation
Finally, stereotaxic procedures are also involved in practices of stem-cell transplantation. The discovery of neural stem cells has opened the door to the possibility of regenerative therapy for many illnesses of the central nervous system, such as Parkinson’s disease. Their properties of self-renewal and multipotency provide the answer to global degeneration or dysfunction, as characterized by the loss of discrete neural populations either isolated or dispersed in specific regions of the brain. Neural stem cell transplantation in the rodent brain allows for further investigation into its therapeutic potential for the treatment of certain CNS diseases (Lee et al., 2008).
The importance of stereotaxy is fully emphasized by the numerous possible applications that it ceaselessly presents. The implications of stereotaxic principles during a technologically-advanced time only add to the many possibilities that this branch of science and these sophisticated set of methods can continue to offer.
Care and Storage
Stereotaxic instruments require appropriate handling and safekeeping for continued use. After every surgical operation, the stereotaxic apparatus along with reusable instruments should be cleaned, decontaminated, and sterilized. The instruments must first be washed with soap and water after use, thoroughly dried, and then autoclaved. More sensitive materials such as cannulae, obturators, and electrodes should be carefully decontaminated or autoclaved, taking into consideration their particular technical tolerances. In cleaning the stereotaxic apparatus, cotton swabs or paper towels soaked with an antiseptic solution of 70% ethyl alcohol are applied to the frame, ear, nose and tooth bars, and verniers, as well as on the heating pad, rectal temperature probe, and workbench. Upon storage, stereotaxic equipment must undergo regular preventive maintenance and service of equipment, depending on their frequency of usage. Measures such as these should effectively maintain the instrument’s precision, as well as prevent the possible stereotaxic error from loose or unsteady components.
References
- Bergeron, M. (2003). Inducing photochemical cortical lesions in rat brain. Current protocols in Neuroscience, 9-16.
- Buchen, L. (2010). Neuroscience: Illuminating the brain. Nature News, 465(7294), 26-28.
- Duncan, R. J. S., Sourkes, T. L., Dubrovsky, B. O., & Quik, M. (1975). Activity Of Aldehyde Dehydrogenase, Aldehyde Reductase, And Acetylcholine Esterase In Stritatum Of Rats Bearing Electrolytic Lesions Of The Medial Forebrain Bundle. Journal of neurochemistry, 24(1), 143-147.
- Ferry, B., Gervasoni, D., & Vogt, C. (2014). Stereotaxic neurosurgery in laboratory rodent: handbook on best practices. Springer Science & Business.
- Fornari, R. V., Wichmann, R., Atsak, P., Atucha, E., Barsegyan, A., Beldjoud, H., … & Roozendaal, B. (2012). Rodent stereotaxic surgery and animal welfare outcome improvements for behavioral neuroscience. Journal of visualized experiments: JoVE, (59).
- Jarrard, L. E. (2002). Use of excitotoxins to lesion the hippocampus: update. Hippocampus, 12(3), 405-414.
- Messier, C., Émond, S., & Ethier, K. (1999). New techniques in stereotaxic surgery and anesthesia in the mouse. Pharmacology Biochemistry and Behavior, 63(2), 313-318.
- Milner, T. A., & Amaral, D. G. (1984). Evidence for a ventral septal projection to the hippocampal formation of the rat. Experimental brain research, 55(3), 579-585.
- Lee, J. P., McKercher, S., Muller, F. J., & Snyder, E. Y. (2008). Neural stem cell transplantation in mouse brain. Current Protocols in Neuroscience, 3-10.
- Li, Y., Peris, J., Zhong, L., & Derendorf, H. (2006). Microdialysis as a tool in local pharmacodynamics. The AAPS journal, 8(2), E222-E235.
- Watson, B.D., Dietrich, W.D., Busto, R., Wachtel, M.S., and Ginsberg, M.D. 1985. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann. Neurol. 17:497-504.
- Zapata, A., Chefer, V. I., & Shippenberg, T. S. (2009). Microdialysis in Rodents. Current Protocols in Neuroscience / Editorial Board, Jacqueline N. Crawley … [et Al.], CHAPTER, Unit 7.2. http://doi.org/10.1002/0471142301.ns0702s47




Reviews
There are no reviews yet.