Spectroscopy refers to the interaction between electromagnetic (EM) radiation and matter as a function of its frequency or wavelength.
From this, a number of quantitative and qualitative measurements defining aspects like sample concentrations, structure, chemical compositions etc, can be obtained depending on the spectroscopic technique and spectrum of EM radiation used.
When EM radiation interacts with matter, its frequency can undergo absorption, diffraction, inelastic scattering, emission and impedance by the atoms of the material substance.
In addition, the interaction increases the internal energy transitions of the atoms and depending on the type of EM radiation, it causes the atoms to rotate in the case of microwave radiation, vibrate for infrared, have electron excitation in case of visible light or undergo ionization with UV and x-ray.
Infrared spectrophotometry, also referred to as Infrared spectroscopy (IR spectroscopy) is one of the most useful techniques for structural and functional group analyses and it has been used widely since its inception to identify unknown substances.
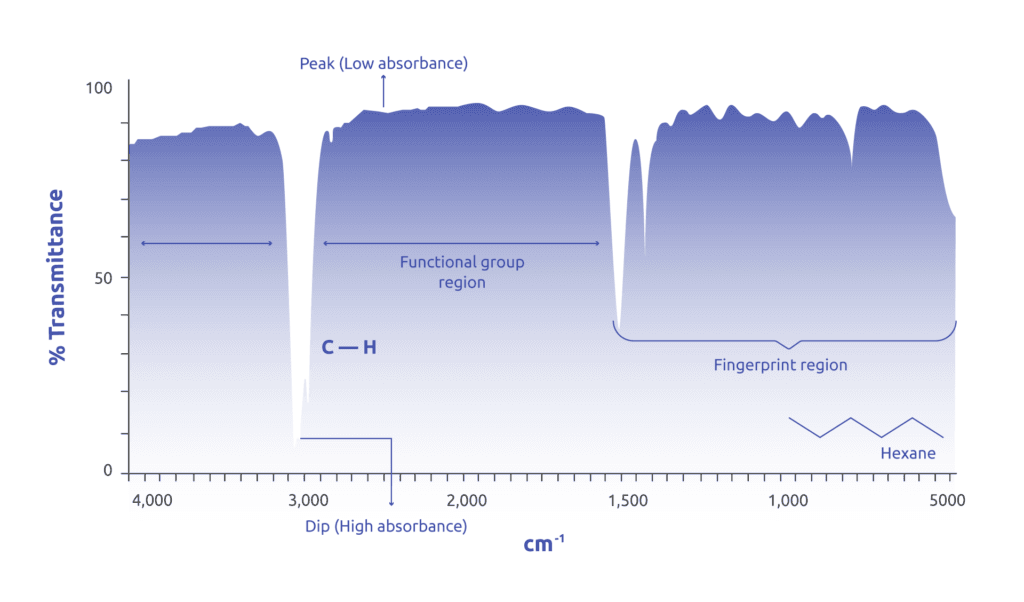
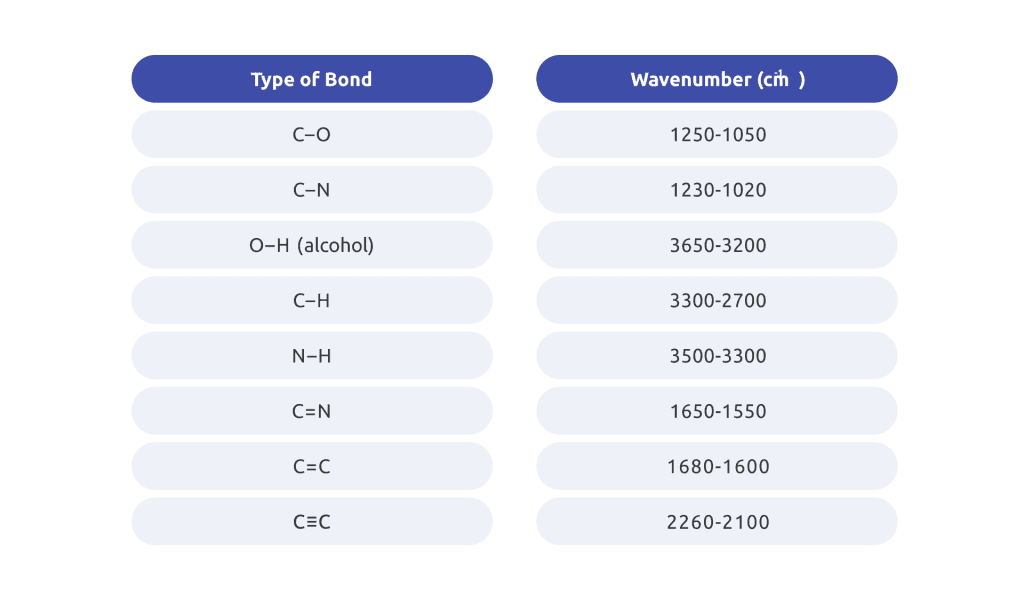
This technique utilizes the ability of atoms to absorb infrared frequencies that match their internal vibrational frequency leading to the generation of an absorption spectra specific to the chemical bonds within the sample under analysis.
The technique has found wide applicability in various fields that require identification of organic substances. In research science, IR spectroscopy has been used in chemistry labs to identify and elucidate the structure of chemical substances.
In industry, it has been used in food and drug administration to investigate the presence of (restricted) substances and for quality control to analyze the purity of food and drugs.
The technique has been used in the manufacturing industry to evaluate ongoing reactions by measuring the appearance or disappearance of particular reactants or detecting the formation of polymers.
Infrared spectrophotometry has also been used in the art world to verify authenticity of prized art by testing paint pigments while in forensic science, it has been used to identify substances of interest from crime scenes.