

Stroke is a leading cause of disability and death worldwide, with over 13.9 million new cases occurring each year.1 Although timely interventions such as thrombolysis and thrombectomy have significantly reduced mortality, acute treatments are limited by narrow therapeutic windows, leaving millions with long-term disabilities.2
Conventional behavioral tests in rodents are widely used to assess outcomes such as neuroprotection, angiogenesis, axonal sprouting, and motor recovery: the rotarod, cylinder, and ladder rung tests.However, these tests often have low reproducibility and capture only limited aspects of sensorimotor function.3
Recent advances in high-speed video recording and deep learning have enabled flexible, transparent, and cost-effective behavioral profiling that overcomes many of these limitations.4
Instead of focusing on any single tool, these approaches use pose estimation and machine learning to automatically track animal movements, extract quantitative features, and uncover subtle behavioral patterns associated with recovery.
This article provides a generalized framework for conducting deep-learning based behavioral profiling in rodent stroke models, focusing on what to report for reproducibility and transparency rather than step-by-step software tutorials.
Understanding behavioral recovery after stroke requires more than simple observation. Traditional scoring systems often capture only overt deficits and fail to detect subtle yet meaningful motor improvements that reflect ongoing neuroplasticity.3,5 Deep learning based behavioral profiling provides a way to detect these minute changes with high accuracy and reproducibility. The workflow involves a series of meticulously linked stages, from inducing a reproducible stroke model to extracting quantitative movement features that track recovery. The process starts with the induction of a focal cortical stroke in rodents using a systematic method such as the photothrombotic stroke model. The photothrombotic stroke model refers to a type of focal ischemic stroke in which a targeted area of the cortex experiences blood flow blockage due to formation of clot locally. This model produces precise cortical damage, allowing correlation of injury with motor deficits such as impaired paw placement, stride length, or coordination. Because of its high reproducibility and low inter-animal variability compared to other models such as middle cerebral artery occlusion (MCAO), It is particularly suitable for computational analysis and ideal for studies involving behavioral change, recovery patterns and neuroplasticity.11-13
After the induction of stroke, the animals are given a recovery period of ideally 3 weeks, during which behavioral testing is conducted at several time points; before the stroke, and on days 3, 7, 14, and 21 after the stroke.3 Such repeated assessments allow the researcher to monitor both immediate and gradual impairments and improvements.
During behavioral testing, animals perform standardized motor tasks that evaluate movement, including gait, balance, and fine motor control in multiple dimensions. Each test is recorded using a high-speed video system under uniform lighting conditions to ensure consistency and reduce artifacts. Accurate and reproducible video recording is essential, as all subsequent computational steps depend on the quality of this raw data.5,6 Multiple camera angles (e.g., lateral and ventral views) allow researchers to visualize the limb movements from all perspectives, allowing analysis of range of movement and the duration it takes for the movement to occur. The recorded videos are then processed using deep learning–based pose estimation algorithms such as ConductVision, DeepLabCut,or SLEAP.4,9,14 These tools allow neural networks to automatically identify and track specific user-defined body parts; such as forepaws, hindpaws, joints, tail, and snout across thousands of frames. This transforms complex data into easy to understand coordinate based datasets, which allows conversion of motion into easily quantifiable information.4 The obtained pose data then serve as the foundation for feature extraction.
In the feature extraction stage, quantitative gait-related parameters are derived from the pose estimation. These features may include stride length, paw placement accuracy, stance and swing durations, inter-limb coordination, and joint angles.6 Each parameter reflects a specific aspect of motor function, and combined they provide a comprehensive behavioral assessment for each animal. Time related changes in these features across days post-stroke show the progression of impairment and recovery, providing higher sensitivity than traditional behavioral scores alone.5
Once features are extracted, machine learning classifiers, such as Random Forests, are automatically applied to classify animals into pre-stroke, post-stroke, and recovery states.5,10 A classifier is an algorithm that observes and identifies data patterns, using these observations to assign data to predefined categories. This allows researchers to trace minute behavioral changes that might otherwise be overlooked in classical scoring. Random Forests can handle complex, messy datasets with many variables and small errors without compromising accuracy, and can highlight which features (e.g., stride length or interlimb coordination) best predict stroke recovery. 5,10
To visualize these high dimensional features (parameters that are challenging to visualize and process due to their number), dimensionality reduction techniques are employed. These techniques reduce the number of input variables in a dataset while retaining as much useful information as possible. Principal Component Analysis (PCA) transforms the dataset into a smaller number of uncorrelated variables, called principal components, which capture the majority of variance in the data. This allows researchers to observe overall patterns and trends in behavioral changes. t-distributed stochastic neighbor embedding (t-SNE), on the other hand, preserves local relationships between data points while projecting them into a low-dimensional space, enabling clear visualization of how individual animals cluster according to behavioral similarity. Using these techniques, animals can be seen progressing from post-stroke clusters toward baseline clusters, representing recovery.5
Integrating classifiers with dimensionality reduction provides a data-driven, objective, and reproducible framework for analyzing functional recovery. This approach captures subtle behavioral trajectories and neuroplastic adaptations that conventional scoring may overlook, supporting more accurate evaluation of therapeutic interventions.4,5,10
Creating a stroke model that is reproducible is of great significance in any behavioral recovery study. Among the available rodent models, the photothrombotic stroke model is widely used for deep learning based behavioral profiling because it produces localized, consistent lesions in the cortex while minimizing mortality.7,8,11 This model closely mimics focal ischemic strokes in humans that affect motor areas, allowing researchers to study post-stroke changes in coordination, balance, and limb use.
1. Principle of Photothrombotic Stroke
The photothrombotic model combines a light-sensitive dye (commonly Rose Bengal) with targeted illumination of a cortical region.7,8 After the activation of the dye by green light (typically 532 nm), reactive oxygen species is generated in the blood vessels which causes endothelial damage and microvascular clot formation. This leads to localized ischemia in the illuminated area, creating a reproducible infarct that affects motor function. Unlike the middle cerebral artery occlusion (MCAO) model, which produces variable infarct sizes and higher mortality, photothrombosis allows precise control over lesion location and size.
2. Experimental Procedure
To prepare the rodents for experiment, they are firstly given anesthesia under continuous monitoring of respiration and body temperature. The body temperature is maintained by a heating pad. Adequate anesthesia is essential to minimize stress in the rodents. Stress minimization is essential as it can affect behavioral outcomes and neuroplasticity.
Rose Bengal is administered at a therapeutically safe dose via intraperitoneal injection.
The infarct size is estimated by the intensity and duration of focused green light illumination applied to the skull over the motor cortex. Standardized experimental settings allow reliable comparison of results between animals.7,11
To confirm the vessel occlusion, Doppler flowmetry and other methods are used to monitor cerebral blood flow, ensuring the target region achieves ischemia.
3. Ethical Considerations
All procedures must be in compliance with the institutional guidelines for animal care and use. Animals should carefully be monitored for signs of pain or distress after stroke, and analgesics should be administered when appropriate. To ensure that, the observed behavioral impairments are due lesion and not as a consequence of procedural stress, adequate recovery time is essential before behavioral testing.8
Behavioral assessments are essential for evaluating functional recovery after stroke because they provide direct insight into the neurological deficits caused by the lesion. To accurately capture these changes, testing is performed over a course of 3 weeks at baseline (pre-stroke) and at multiple post-stroke time points, commonly on days 3, 7, 14, and 21. Baseline measurements allow each animal’s normal performance to be established, while repeated assessments track the pattern of recovery, revealing both early impairments and gradual improvements as a result of cortical reorganization and neuroplasticity.3,5
A combination of relevant behavioral tests is used to evaluate different aspects of motor and sensorimotor function in the rodents. The rotarod test measures motor coordination, balance, and endurance by recording the time taken by the rodents to fall from a rotating rod as stroke often impairs balance and fine motor control, tracking changes in latency to fall over time provides an objective measure of recovery. The cylinder test measures forelimb asymmetry during spontaneous rearing, showing how the animal compensates for weakness in the affected limb. Recovery can be traced by the gradual restoration of symmetric limb use.3
The ladder rung walking test evaluates skilled paw placement and inter-limb coordination as animals climb a horizontal ladder with irregularly spaced rungs. Even subtle deficits in precision or timing can be detected, making this test particularly sensitive for identifying fine motor impairments. Similarly, the runway or open-field test captures spontaneous locomotion, including gait speed, distance, and symmetry, offering a broader assessment of general motor function. The single-pellet reaching task focuses on goal-directed forelimb movements, quantifying dexterity and coordination, which are often disrupted by cortical lesions but can recover with neuroplastic reorganization.3,5
All behavioral tests are recorded using high-speed cameras under uniform lighting, ensuring that every movement is captured with sufficient resolution for computational analysis. Controlled testing environments minimize stress which in turn reduces variability and ensures that the measured changes reflect true recovery rather than environmental factors. Repeating trials for each test allows for averaging and more reliable assessment.4,5
High-quality video acquisition is a crucial step in behavioral profiling, as it provides the raw data from which all computational analyses are derived. Accurate recordings ensure the detection and quantification of subtle motor deficits; conversely, poor-quality videos can introduce errors and reduce reproducibility. To achieve this, all behavioral tests are recorded using high-speed cameras, typically at 60 frames per second or higher, which allows precise tracking of rapid limb movements and joint articulations.4
Videos are captured from multiple angles, such as lateral and ventral views, and may utilize angled mirrors to simultaneously record bottom and side perspectives. This multi-angle approach ensures overall coverage of the animal’s movements, providing detailed information about stride, paw placement, and inter-limb coordination that could otherwise be missed from a single viewpoint. Consistent lighting and a uniform background are also critical, as shadows or reflections can interfere with pose estimation algorithms reducing tracking accuracy.
Each trial is stored as a raw video file (MP4 or AVI) without compression which could otherwise degrade image quality, to ensure reproducibility. The camera placement, framing, and environmental conditions across sessions must be constant to reduce variability, to ensure that any observed differences in behavior reflect true changes in motor function rather than experimental artifacts. Proper video acquisition is essential not only to facilitate accurate pose estimation but also to allow the extracted features to be reliably compared across different time points, animals, and experimental conditions.
By carefully controlling all aspects of video recording, researchers can generate datasets that are suitable for deep learning based behavioral profiling, enabling sensitive, quantitative, and reproducible assessment of post-stroke recovery.5
Pose estimation transforms raw video recordings into quantitative data by tracking the positions of specific body parts over time. This step is crucial as it converts complex, high-speed movements into measurable parameters that can be objectively analysed, which overcomes the subjectivity and limitations of the traditional behavioral scoring.4,9
Deep learning–based pose estimation algorithms, such as DeepLabCut, SLEAP, or ConductVision, use convolutional neural networks to detect and track user-defined body parts, including forepaws, hindpaws, joints, snout, and tail. Representative frames from the recorded videos are first selected and manually labeled to teach the algorithm what each body part looks like under various postures and lighting conditions. By training on these labeled frames, the network learns to recognize the same features in new, unlabeled videos.4,14
Accuracy is refined through iterative review and correction of mislabeled frames, ensuring the model predicts the positions of each body part even in complex or occluded movements reliably. Once trained, the model produces precise x and y coordinates for every labeled point across all frames, creating a rich dataset of movement trajectories. This process allows researchers to quantify subtle changes in gait, limb coordination, and joint angles that may be invisible to human observers.5
Pose estimation is particularly valuable because it provides high-resolution time related and location related data, capturing not only gross motor deficits but also minute variations in movement patterns that indicate recovery or compensation. By converting visual behavior into numeric data, it establishes a foundation for subsequent feature extraction and machine learning analysis, enabling objective, reproducible, and detailed assessment of post-stroke motor function.
Once pose estimation has generated coordinate data for each body part across frames, the next step is to extract meaningful features to quantify motor performance. This process translates raw information about the rodent’s position into kinematic and gait parameters, providing quantifiable measures of functional recovery that extend beyond classical observational scoring.5,6
From runway or bottom-view videos, features such as stride length, stride speed, stance and swing durations, and left–right coordination can be calculated. These metrics analyze gross locomotor function to reveal any minute asymmetries or delays in movement that may indicate post- stroke deformities. Side-view or ladder rung recordings allow extraction of paw placement accuracy, slip frequency, and inter-joint angles, capturing of fine motor control and limb coordination.
Joint-specific kinematics, including range of motion, angular variability, and protraction/retraction amplitude, provide insight into how overall movement is influenced by individual limbs. In order to highlight recovery and persistent deficits observing changes in these parameters over time, indicates neuroplastic adaptation that often remains unnoticed with classical behavioral tests.6
Typically, hundreds of features can be extracted per animal, depending on the number of labeled joints, frame rate, and duration of the recordings. Such high-dimensional datasets allow detailed comparisons between pre-stroke, post-stroke, and recovery points of time, facilitating a thorough understanding of motor recovery. Importantly, consistent feature extraction across animals and trials ensures reproducibility and allows for reliable application of machine learning classifiers in subsequent analyses.5
By converting raw positional data into a structured set of kinematic parameters, feature extraction bridges the gap between behavioral observation and quantitative analysis, forming a foundation for data-driven evaluation of stroke recovery.
After extraction of kinematic and gait features from pose estimation data, machine learning techniques are implemented to identify impairment and recovery patterns that may be inapparent in conventional scoring. Using these algorithms allows researchers to handle the high-dimensional datasets generated during feature extraction, trace subtle behavioral differences, and quantify course of recovery objectively.5
Supervised classifiers, such as Random Forests, can be trained on labeled datasets to determine which features are most predictive of stroke-induced deficits or recovery. This approach not only highlights the most relevant parameters but also enables automated scoring of new animals, increasing efficiency and reproducibility. Dimensionality reduction techniques, such as Principal Component Analysis (PCA) or t-distributed Stochastic Neighbor Embedding (t-SNE), are often applied to visualize relationships between pre-stroke, post-stroke, and recovery datasets. These visualizations can reveal clustering of behavioral patterns among animals showing similar motor abilities at a given point of time, illustrating how animals gradually return to pre-stroke motor function as neuroplasticity occurs.
Comparing machine learning outputs to traditional behavioral scores, such as rotarod latency or cylinder asymmetry, validates the sensitivity and robustness of the computational approach. Moreover, subtle changes in stride variability, inter-limb coordination, or joint kinematics identified through machine learning may serve as early biomarkers of recovery, which can inform both experimental interventions and translational research in humans.5
By integrating feature extraction with advanced computational analysis, machine learning based profiling provides a quantitative, reproducible, and comprehensive assessment of post-stroke motor recovery, uncovering insights that would be difficult or impossible to capture using classical behavioral tests alone.
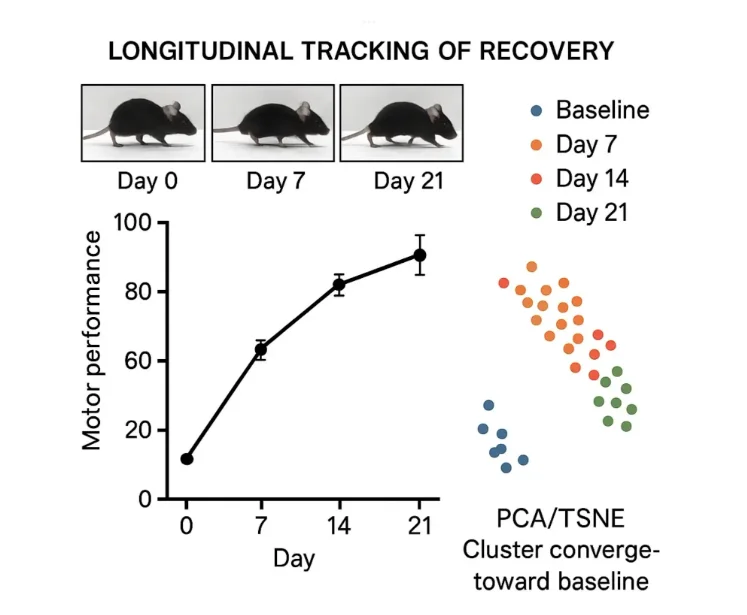
Deep learning-based behavioral profiling offers a detailed and quantitative view of post-stroke recovery that complements traditional behavioral assessments. Researchers can expect to observe progressive improvements in motor function over time, reflected in both conventional scores and machine learning derived features. Subtle impairments, such as changes in stance duration, stride variability, or inter-limb coordination, may be detectable even before obvious recovery is observed in gross motor tests.5
Visualization techniques, such as PCA or t-SNE plots, typically show clustering of data points corresponding to pre-stroke, post-stroke, and recovery phases. Early post-stroke data may cluster separately from baseline, reflecting acute motor deficits, while later time points gradually shift toward baseline clusters, illustrating functional recovery and cortical reorganization. These patterns provide insight into neuroplastic processes underlying motor improvement and help quantify the effectiveness of interventions.
Feature-level analysis can reveal which specific aspects of movement recover first, which remain impaired, and how compensatory strategies may develop. For example, fine motor control in the single-pellet reaching task may lag behind improvements in gross locomotion, indicating selective cortical or corticospinal recovery. By integrating multiple behavioral parameters, researchers gain a comprehensive understanding of both the extent and quality of recovery, rather than relying on a single outcome measure.
Overall, deep learning–based behavioral profiling enables sensitive, reproducible, and interpretable assessments of stroke recovery, supporting robust evaluation of new therapeutic interventions and providing insights that are challenging to obtain through conventional testing alone.7
Datasets from pose estimation and machine learning pipelines can be stored in interoperable formats such as Neurodata Without Borders (NWB) an initiative by The Kavli Foundation (2014) that standardizes neuroscience data globally. By providing a common structure for electrophysiology, imaging, and behavioral tracking data, NWB enables seamless sharing, analysis, and long-term preservation. Standardized, shareable behavioral datasets promote FAIR (Findable, Accessible, Interoperable, Reusable) data practices ensuring reproducibility and cross-lab validation. Incorporating detailed metadata such as animal characteristics, recording parameters, and feature definitions allows consistent interpretation and comparison across studies. This structured approach enhances the translational value of behavioral research, aligning rodent models of stroke recovery with human rehabilitation datasets and AI-driven gait tracking. Outputs from these pipelines can be easily packaged for sharing in formats like .nwb; for in-depth strategies on open-data implementation, refer to Article 2 in this series.
Written by researchers, for researchers — powered by Conduct Science.










ConductScience Fellow, is a Nepali medical student exploring gait analysis, brain–machine interfaces, and AI-based behavioral assessment for neurological applications.
