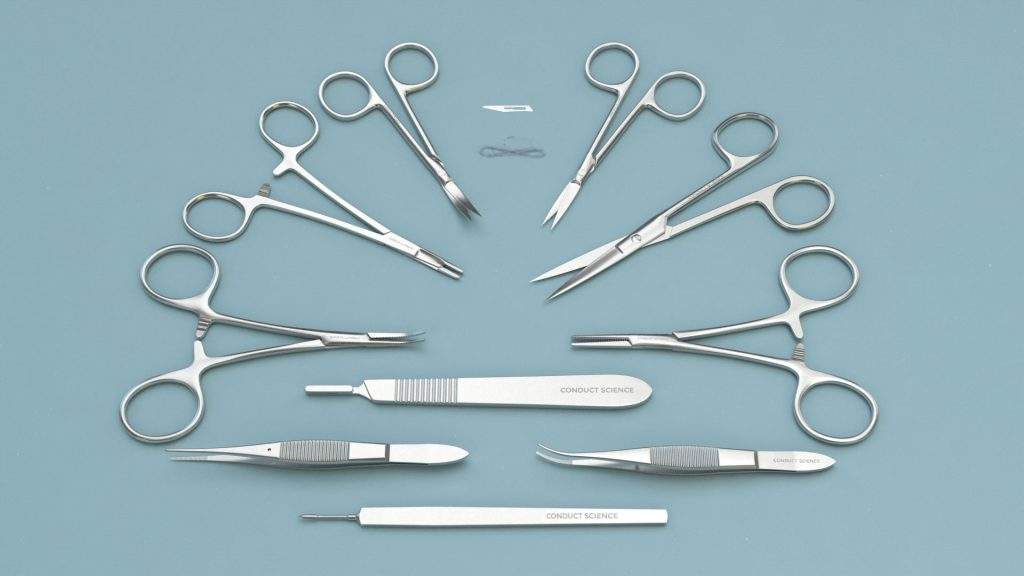
Mouse Kit
| Cat.No. |
Product Description |
Qty |
| S31011-01 |
11# Scalpel Blades (Box of 100pcs) |
1 |
| S32001-12 |
Scalpel Handles 3# Solid-12cm |
1 |
| S33007-12 |
ZIEGLER Knives-4mm Cutting |
1 |
| S12003-09 |
IRIS-Fine Scissors (Round Type)-S/S Str/9.5cm |
1 |
| S12004-09 |
IRIS-Fine Scissors (Round Type)-S/S Cvd/9.5cm |
1 |
| S14014-11 |
Operating Scissors (Round Type)-S/S Str/11.5cm |
1 |
| F12005-10 |
IRIS Dissecting Forceps-Str, 0.8mm Tips, 10cm |
2 |
| F12006-10 |
Forceps-Light Cvd, 0.8mm Tips, 10cm |
1 |
| F22002-10 |
HARTMAN Mosquito Forceps-Str, 1.0mm Tips, 10cm |
2 |
| F31047-12 |
Needle Holders with Scissors-Str, 12cm |
1 |
| F35205-60 |
Sutures w/Needle-△3/8/2.5×7/30㎝/6-0 (50/Box) |
10 |
| F22003-10 |
HARTMAN Mosquito Forceps-Cvd, 1.0mm Tips, 10cm |
2 |
| SP0000-P |
Instrument Storage Portfolio,32*22cm |
1 |
Rat Kit
| Cat.No. |
Product Description |
Qty |
| S31011-01 |
11# Scalpel Blades (Box of 100pcs) |
1 |
| S32001-12 |
Scalpel Handles 3# Solid-12cm |
1 |
| S33007-12 |
ZIEGLER Knives-4mm Cutting |
1 |
| S12005-10 |
IRIS-Fine Scissors (Round Type)-S/S Str/10.5cm |
1 |
| S12006-10 |
IRIS-Fine Scissors (Round Type)-S/S Cvd/10.5cm |
1 |
| S14014-12 |
Operating Scissors (Round Type)-S/S Str/12.5cm |
1 |
| F12010-10 |
Dressing Forceps-Str, 1.9mm Tips, 10.5cm |
2 |
| F12011-10 |
Dressing Forceps-Cvd, 1.9mm Tips, 10.5cm |
1 |
| F22006-12 |
HALSTED Mosquito Forceps-Str, 1.0mm Tips, 12.5cm |
2 |
| F31047-12 |
OLSEN-HEGAR Needle Holders with Scissors-Str, 12cm |
1 |
| F35205-60 |
w/Needle-△3/8/2.5×7/30 /6-0 (50/Box) |
10 |
| R22009-01 |
ALM 4x4 Teeth Retractors-Blunt, 7cm |
1 |
| F22007-12 |
HALSTED Mosquito Forceps-Cvd, 1.0mm Tips, 12.5cm |
2 |
| SP0000-P |
Instrument Storage Portfolio, 32*22cm |
1 |
Introduction
A brain microdialysis surgical kit is used for intracerebral microdialysis. Intracerebral microdialysis is a surgical technique primarily used for in vivo sampling of neurotransmitters. The method is widely used in neuroscience for sampling, assessment, and quantification of neuropeptides, hormones, drugs, and other molecules in the brain, periphery, and interstitial fluid. The technique is also applied for the investigation of the pharmacological effects of potent drugs on amino acid and monoamine neurotransmitters.
There are two basic types of brain microdialysis experiments namely conventional microdialysis and no-net-flux microdialysis. In the conventional microdialysis, neurotransmitter-free artificial cerebrospinal fluid (aCSF) is perfused through the cannula, and the neurotransmitter is collected in the dialysate. And, in the no-net-flux or zero-net-flux method, aCSF with several different concentrations of neurotransmitter is pushed through the probe and the amount of the analyte of interest increased or decreased in the probe is measured (
Vladimir, Alexis, Agustin, & Shippenberg, 2009). Both methods are applied for brain microdialysis in rodents.
Microdialysis is prominently used for sampling of molecules from several organ systems including the blood, muscles, liver, eyes, etc. The history of the use of microdialysis as a sampling technique dates back to 1960s when it began with the push-pull method, which used a semi-permeable membrane to sample electrolytes and amino acids from the neuronal extracellular fluid. The development of the dialysis bag further developed the technique for sampling. The quantification, characterization, and sampling of neuropeptides and neurotransmitters in awake-freely-moving laboratory animals are the most widely used applications of brain microdialysis.
The microdialysis sampling technique is based on the law of diffusion. The law of diffusion explains the passive movement of molecules across the concentration gradient developed between the membrane and the interstitial fluid. The microdialysis procedure is an interchange between the dialysis membrane with “the probe,” the perfused liquid, the target tissue or organ, and the interstitial or extracellular fluid. An equilibrating fluid is perfused in the membrane tissue fluid to outside the membrane (
Darvesh et al., 2011). The molecules of interest such as electrolytes, neuropeptides, amino acids, hormones, neurotransmitters, or neuromodulators, etc. are present in the dialysate outflow after equilibration.
Surgical microscope
Surgical tools and supplies
Preoperative Set-Up and Anesthesia Induction
Prior to the surgical procedure, examine the animals physically. Monitor the animals for nutritional status, fur quality (thinning, dirty), and behavior (limbs and trunk movement, abnormal gait, rigid walking, and a flat abdomen). Also, inspect the natural orifices for discharge from the nose, increased salivation, and filths around the anus and genitals, and observe the condition of the eyes. Monitor the breathing pattern of the subjects because non-manifesting subclinical pulmonary diseases may lead to severe respiratory failure following general anesthesia with subsequent death of the animal.
Anesthetize the animals with halothane (1.5-2% in a 50:50 O2/NzO mixture). After anesthesia induction, asses the depth of the anesthesia using the toe pinch test. Observe and assess the subjects for physiological parameters throughout the experimental procedures to ensure that the anesthesia is effective.
Brain Microdialysis Procedure
- Place the subject in the stereotaxic frame and set the incisor bar at 3.3 mm.
- Implant a semi-permeable membrane-containing probe or guide cannula in the ventral hippocampus.
- Allow the animals to recover for 24 hours.
- Pump the perfusion fluid into the probe via a perfusion pump slowly at an optimal rate (generally 1.8 – 2.2 μl/min) and collect the dialysate with the help of the collection device after equilibration.
- Quantify and characterize the analyte as per experimental needs.
Post-Operative Care and Pain Management
Recover the animals on a flat paper bedding (sterile paper towels, etc.). For a speedy recovery, keep the animals warm. Place the recovery cage half-on a
heating pad so that animals can choose their preferred temperature as they recover from the anesthesia. The animals which underwent surgery must have regained the ability to move in the cage freely. Monitor the animals post-operatively for unexpected signs of illness and pain. To regain weight quickly, provide the animals with proper analgesia and food.
Keep the animals under observation for five to seven days after surgery. Animals should be bright, alert, and active after the recovery. The animals should generally be interacting with the cage mates, eating and drinking. Depression, anorexia, or sluggishness indicate abnormal behavior. Food/fluid intake is essential to recovery. Provide animals with easier access to food and water. Inflammation, redness, swelling, discharge (purulent or serious), pain, anxiety or the opening of the incision (dehiscence) are the signs of inflammation. Treat the symptoms with proper ointment.
Applications
Comparison of the effects of intra-cerebrally administered MPP + (1-methyl-4-phenylpyridinium) in mouse and rat: microdialysis of dopamine and metabolites (Rollema et al., 1989)
Intracerebral microdialysis was used to measure the basal output of dihydroxyphenylacetic acid (DOPAC), Dopamine (DA), homovanillic acid (HVA) and 5-hydroxyindole-acetic acid (5-HIAA) from rat and mouse striatum in vivo. DOPAC/HVA ratios in the output dialysates from the mouse and rat striatum were 1:2 respectively. It was observed that the extracellular dopamine levels were 3 times lower than the level in the tissue concentrations. Similar effects of the intra-cerebrally administered dopaminergic neurotoxin l-methyl-4-phenylpyridinium (MPP +) were seen in both the subjects. The metabolites output accompanied the immediate and massive release of dopamine. At 5-12 h after MPP + administration the basal dopamine release was not detectable, and the subsequent MPP ~ perfusion did not potentiate the dopamine release. The brain microdialysis enabled the researchers to compare and analyze the effects of the test molecules in rodents.
Assessment of changes in 5-HT release in the ventral hippocampus in rats (
Wright, Upton, & Marsden., 1992)
The study was conducted to combine in vivo microdialysis with behavior on the elevated X-maze in Sprague-Dawley rats to determine the levels of 5-HT release in the ventral hippocampus. Brain microdialysis was used to sample the 5-HT and 5-HIAA. The subjects were exposed to elevated X-maze for 20 minutes. An increase in the extracellular 5-HT in the ventral hippocampus was observed. However, there was no change in the level of extracellular 5-HIAA. The release of 5-HT in the extracellular was increased when the rats were exposed to either the closed or the open arms of the elevated X-maze, however, the 5- HT levels were not significantly increased when the subjects were restricted to the open arms as compared to the closed arms restriction. Diazepam (2.5 mg kg- 1 IP) treatment significantly reduced the level of extracellular 5-HT in the ventral hippocampus and induced anxiety over 5 min and 20 min X-maze exposure. The results suggested that the 5-HTIA receptor partial agonist ipsapirone (1 mgkg -1 IP) inhibits the release of extracellular 5-HT in the ventral hippocampus but does not has any effect on the animal’s behavior. The novel anxiolytic F2692 (10mgkg -1 IP) was found as antagonistic to the increase in extracellular 5-HT in the ventral hippocampus and induced anxiety over the 5 min but not in the 20 min period on the X-maze. The brain microdialysis enabled the measurement and characterization of the anxiolytic compounds on extracellular 5- HT levels and helped to delineate their behavioral profile on the X-maze.
Evaluation of the basal levels of neurotransmitters in the brain extracellular fluid (
Boschi, Launay, Rips, & Scherrmann, 1995)
Brain microdialysis has been the feasible technique to sample the neurotransmitters from small brain areas of OF1 (iffa Credo mice) mice. In the study, dorsal hippocampus and nucleus accumbens were the brain regions of interest. Using the brain microdialysis, biogenic amine metabolites were quantified in the dialysate samples and were measured then by high-performance liquid chromatography (HPLC) accompanying the electrochemical detection (ED). DOPAC (3,4-Dihydroxyphenylacetic acid), MHPG (3-Methoxy-4-Hydroxyphenylglycol), and HVA (homovanillic acid) in the dorsal hippocampus were obtained soon after the probe was inserted, whereas 5-HIAA (5-Hydroxytryptamine indole acetic acid) concentration declined gradually. After 80 minutes the levels of DOPAC, HVA, and 5HIAA were stabilized in the nucleus accumbens. The compounds collected from the nucleus accumbens could be used for the assessment of drug-induced interactions. The brain microdialysis allowed the measurement of the basal levels of neurotransmitters and could allow the correlation of biochemical changes and pharmacological effects. The method can also facilitate further pharmacokinetic and biochemical research in rodents.
Assessment of the effects of aripiprazole on dopaminergic and serotonergic systems in rodents (
Bortolozzi et al., 2007)
In the study, the in vivo effects of aripiprazole on serotonergic and dopaminergic systems were evaluated using the brain microdialysis. The research was conducted using male albino Wistar rats and C57BL/6 mice. It was observed that the 5-HT (5-hydroxytryptamine output was reduced in the medial prefrontal cortex (mPFC) of the hippocampus and the dorsal raphe nucleus in the rat following aripiprazole systemic administration. Also, the extracellular levels of 5-HT were reduced in the mPFC of wild-type (WT) but not in the 5-HT1A (−/−) knockout (KO) mice after the aripiprazole administration. The results suggested that aripiprazole has a reversal effect on extracellular 5-HT output potentiated by the 5-HT2A/2C receptor agonist DOI local application in mPFC. However, dopamine output in mPFC of WT was increased because of the aripiprazole. Whereas, the dopamine level was not increased in the 5-HT1A KO mice. In contrast to these, the haloperidol increases the activation of dopamine neurons in the ventral tegmental region of the brain. The dopaminergic activity was moderately reduced following the aripiprazole administration. The study indicates that the aripiprazole regulates the in vivo 5-HT and DA release in the medial prefrontal cortex by activating the 5-HT1A receptors. It was also concluded that the aripiprazole serves as a partial agonist at dopamine D2 autoreceptors, an action that is contrasting to the effects of haloperidol. The brain microdialysis technique is a feasible and reliable method to delineate the in vivo effects of atypical antipsychotic drugs.
Precautions
Do not exceed pre-anesthetic fasting beyond 2 hours because of the high metabolic rate of the rodents as extended food deprivation can disturb the balance leading to the metabolic acidosis, and hypoglycemia. Handle the animals gently to avoid stress which may lead to cardiac arrest following tachyarrhythmia during the general anesthesia. Also, the strain and breed of the laboratory animal must be selected as per the experimental requirements. During the surgical procedures, take care not to damage the surrounding tissues and muscles. Use clean home cages for animals to avoid contaminating the surgery area.
Summary
- In vivo brain microdialysis is a quantification and sampling technique used to measure the levels of the neurotransmitters in the brain extracellular fluid.
- Brain microdialysis has been widely used to evaluate the physiological and pharmacological effects of potential drugs in the extra-neuronal fluid.
- Rodent brain in vivo microdialysis has also been employed to assess the region-specific neurochemical changes induced by psychotic drugs and pharmacological compounds in the freely moving and awake rodents.
- The technique has enabled the researchers to measure the changes in concentrations of neurotransmitters and their respective metabolites following the drug administration.
- In vivo microdialysis helps to characterize the neuropharmacological agents such as the drugs of abuse, antidepressants, and antipsychotic agents.
- In vivo brain microdialysis has been considered as the backbone of neurological disorders treatment.
References
- Bortolozzi, A., Díaz-Mataix, L., Toth, M., Celada, P., & Artigas, F. (2007). In vivo actions of aripiprazole on serotonergic and dopaminergic systems in rodent brain. Psychopharmacology (Berl), 191(3), 745-58.
- Boschi, G., Launay, N., Rips, R., & Scherrmann, J. M. (1995). Brain microdialysis in the mouse. J Pharmacol Toxicol Methods, 33(1), 29-33.
- Darvesh, A. S., Carroll, R. T., Geldenhuys, W. J., Gudelsky, G. A., Klein, J., Meshul, C. K., & Schyf, C. J. (2011). In vivo brain microdialysis: advances in neuropsychopharmacology and drug discovery. Expert Opin Drug Discov. 2011, 6(2), 109-127.
- Rollema, H., Alexander, G. M., Grothusen, J. R., Matos, F. F., & Castagnoli, N. (1989). Comparison of the effects of intracerebrally administered MPP+ (1-methyl-4-phenylpyridinium) in three species: microdialysis of dopamine and metabolites in mouse, rat and monkey striatum. Neurosci Lett., 106(3), 275-81.
- Vladimir, I. C., Alexis, C. T., Agustin, Z., & Shippenberg, T. S. (2009). Overview of Brain Microdialysis. Curr Protoc Neurosci.
- Wright, I. K., Upton, N., & Marsden., C. A. (1992). Effect of established and putative anxiolytics on extracellular 5-HT and 5-HIAA in the ventral hippocampus of rats during behaviour on the elevated X-maze. Psychopharmacology (Berl)., 109(3), 338-46.