
Have you ever thought about why athletes or gymnasts are often on a protein-rich diet? It’s because these people expend more energy compared to people who don’t work out. And a diet rich in proteins fuels the body to build muscle, promote quick recovery, boost immunity, replenish glycogen, and burn fat, all of which are important during strenuous workouts.[1]
But are they only required for people who work out? No, protein has tons of roles to play in all living organisms. It’s one of the essential macronutrients synthesized by organisms required for a healthy life.
This article is all about what proteins are, their types, and how they are different from one another. It also covers the functions of proteins in living organisms and methods to study these molecules in lab conditions.
Proteins are one of the versatile macromolecules in living organisms that serve crucial functions in different biological processes.
They are present throughout the body of organisms — in muscles, bones, skin, hair, and virtually every other body part or tissue.[2] They are made of 20 amino acids, arranged in different structural forms, forming around 10,000 proteins (or even more than that).[2]
Some key properties of proteins responsible for their functions are:[3]
Proteins are constructed linearly from 20 amino acids, which is a recurring process in a cell. But the linear/primary sequence of the protein is not responsible for their functional roles.[3]
The sequences spontaneously fold into different arrangements, forming three-dimensional structures that are determined by the amino acids present in the sequence. And these folded structures facilitate the functions of these proteins.[3]
The most predominant functional groups present in proteins are alcohols, thiols, thioethers, carboxylic acids, carboxamides, and a variety of basic groups.[3]
These functional groups combined in different random patterns in the amino acid sequence accounts for the broad spectrum of protein function. In simple words, the properties of the functional groups of proteins determine their enzymatic and other metabolic functions in the body.[3]
Proteins interact with other proteins or biomolecules to perform tasks that they alone aren’t equipped for. Examples of such tasks are DNA replication, the intracellular transmission of signals, and other complex essential biological processes.[3]
The rigid proteins (have tightly compact structures which restrict any motion or movement) are involved in the structural formation of cells, such as the cytoskeleton which forms internal scaffolding within cells.[3]
Whereas flexible proteins (have loosely bound structures that can easily rearrange or move when required) act as hinges, springs, and levers to perform essential functions and form complexes with other proteins and macromolecules.[3]
The process of protein synthesis is called translation. In this process, the mRNA codes are translated into the respective amino acids that are involved in protein formation. Each amino acid has its unique nucleotide sequence of genes. The genetic code of one amino acid consists of three sets of nucleotides called codons.[4]
The process starts with the transcription of DNA to pre-mRNA using RNA polymerase. The pre-mRNA is then modified through post-transcription to form a mature mRNA.[4] The mature mRNA is used as a template by ribosomes for protein synthesis.
Ribosomes bind to the mRNA and use one codon (consisting of three nucleotides) matching the anticodon present on tRNA (transfer ribonucleic acid is an adaptor molecule that helps in decoding mRNA) for the synthesis of one amino acid.[4] The four nucleotides, A (adenine), G (guanine), C (cytosine), and T (thymine) form a different combination of nucleotides to form different amino acids.

Figure: An illustration of protein synthesis.
The chemical synthesis of proteins involves peptide synthesis, which uses strategies like chemical ligation, Staudinger ligation, or other orthogonal chemical reactions to couple synthetic peptides.[4] Here, peptides (a chain of 30-50 amino acids) are produced and linked together via amide or peptide bonds to form specific proteins.
But it’s an inefficient technique when it comes to producing a polypeptide chain of more than 300 amino acids. It’s important to note that the chemical synthesis of protein occurs from C-terminus to N-terminus, whereas in biological processes, the synthesis occurs in the opposite direction, that is from N-terminus to C-terminus.[4]
Proteins are built by linking two or more amino acid residues together in different orientations or configurations. The amino acids in the proteins are joined together by peptide bonds formed through a condensation reaction between two amino acids, releasing water molecules in the process.
The description of protein structure may sound similar to peptides, but there is a fine line between proteins and peptides as both differ in size, structure, and functions, as explained below:
The different levels of protein structure are possible as a result of different chemical interactions within its structure. For example, the folding of the linear sequence of amino acids into three-dimensional structures is driven by several non-covalent interactions such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic interactions.
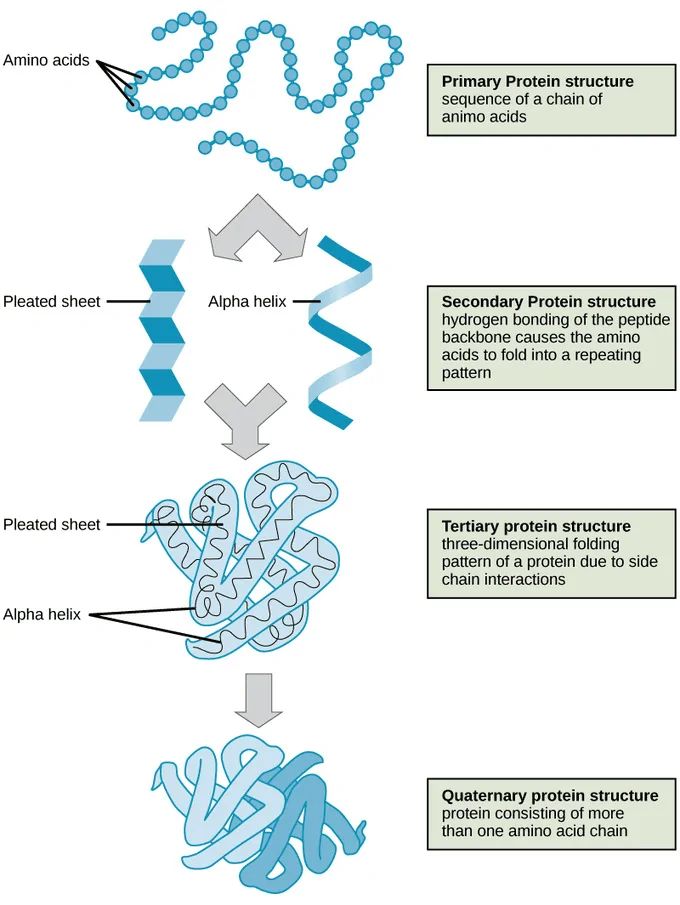
Given below is a short description of the four levels of protein structure.[6]
The primary structure is the arrangement of amino acids into a linear polypeptide chain. Here, the amino acids are only joined by peptide bonds and disulfide bonds. The sequence of amino acids in a polypeptide chain is responsible for the functions of the proteins, which is decided by decoding the genetic codes or genes corresponding to the proteins.[6]

Secondary structure is the regular, recurring arrangements of adjacent amino acids in a polypeptide chain.[7] This occurs by forming hydrogen bonds between molecules and it represents the initial stage of polypeptides folding into three-dimensional structural form, that is, before tertiary and quaternary structures.
The most common secondary structures are alpha-helix and beta-sheet.
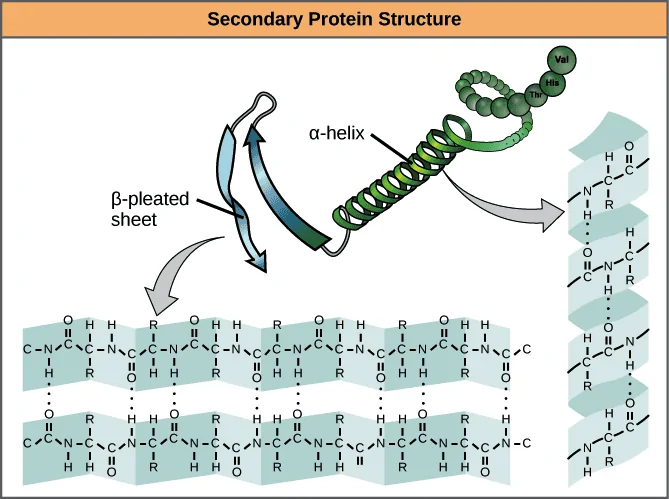
Here, the polypeptide chain further folds into a three-dimensional space, involving one or several domains. The alpha-helix and beta sheets are folded into a compact globular structure that’s driven by non-specific hydrophobic interactions.
The interactions occur between polar, nonpolar, acidic, and basic R groups within the polypeptide chain.[7] When proteins lose the tertiary structural form, they can’t perform any of their functions.
In an aqueous environment, the hydrophobic or nonpolar groups move into the interior of the proteins while the hydrophilic R groups lie on the surface or outside the structure.[7]
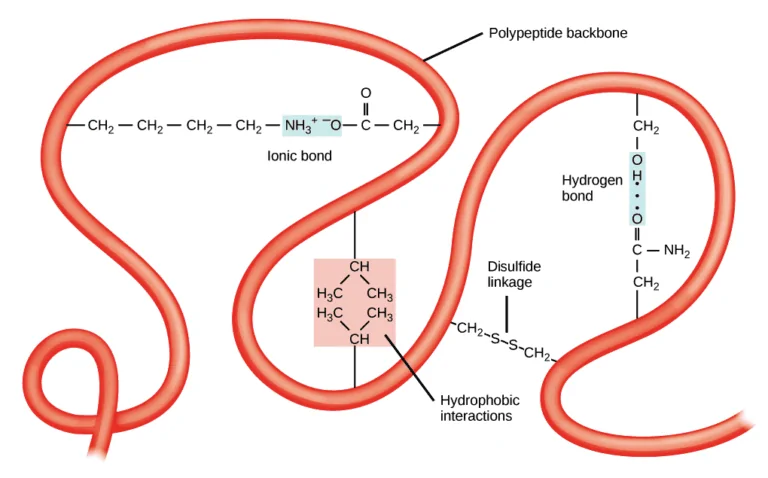
More than one polypeptide chain is involved in the formation of a quaternary structure. The polypeptide chains can be identical multiple copies or different in amino acid sequences. It’s the weak interactions such as hydrogen bonding and London dispersion forces, that keep the multiple polypeptides bound together in forming a quaternary structure.[6]
A well-known example of a quaternary structure is hemoglobin that carries oxygen in the blood and consists of four peptide chains (two alpha and two beta chains) to form a tetramer.[6]
Proteins perform several essential life-sustaining metabolic functions in organisms. Based on their functions, they are classified into:[12]
Different characteristics of proteins are studied using different techniques which include: in vivo, in vitro, and in silico.[4]
In vivo techniques are used to study the functional roles of proteins inside cells; in vitro techniques are preferred when it comes to understanding the working mechanism of proteins in a certain environment; while in silico is a computation method of studying proteins, like understanding protein complex formation and structural determination.[4]
Given below are techniques based on the studies they are used for:[4]
Proteins are one of the essential biomolecules required to sustain life. They consist of amino acids arranged in four structural levels: primary, secondary, tertiary, and quaternary.
They differ from peptides at both structural and functional levels. The functions of proteins range from transportation of molecules, structural formation, to storage and enzymatic roles, whereas peptides only influence the activities of other molecules.
The spectrum of functions of proteins in organisms has pulled the scientist’s brain towards the complexity of their actions, hence scientists are curious to understand the structure and working mechanism of the molecule. Some common tools used to study proteins are mass spectrometry, chromatography, circular dichroism, and spectrometry.
Working with proteins requires a high level of precaution and expertise. And considering its importance in organisms, scientists are trying to develop, synthesize, and purify the molecule at an individual level for application in medical areas. The area of proteomics also opens the doors for young scientists to make a breakthrough and contribute to enhancing the quality of human life.
