
The Bussey-Saksida Touch Screen Operant Conditioning Chamber is a multi-paradigm device used to study operant conditioning in rodents. It features a large, high-resolution touchscreen that serves as the primary input/output interface for different tasks. This touch screen presents various visual stimuli, respond to animal touch inputs, and log their responses.
Additionally, the chamber includes operant conditioning tools like levers, nose pokes and food dispensers or lickometers, which are used to reward subjects for specific behaviors, such as pressing a button or touching a designated area on the screen.
The chamber is designed with a range of environmental controls and cues, including, lighting and sound. These settings can be customized to your experimental needs. Researchers can also program the chamber to create and control complex tasks and training programs, such as visual discrimination, memory tests, and attention tasks. The system automatically records various behavioral metrics, such as response times and accuracy, and stores detailed data for analysis.
The chamber is also valuable for pharmacological studies, as it enables researchers to investigate how various substances or treatments, such as drugs, affect behavior and cognitive abilities. In the field of comparative neuroscience, the device allows for the comparison of behavior across different species, offering insights into animal cognition and the neurological foundations of behavior.
| Mice | Rat |
| Chamber size: 20cm (l) x 20cm (w) x 20cm (h) | Chamber size: 30cm (l) x 30cm (w) x 30cm (h) |
| (1x) Touchscreen | (1x) Touchscreen |
| (1x) Pellet Dispenser (or Lickometer) | (1x) Pellet Dispenser (or Lickometer) |
| (1x) Pellet Receptacle | (1x) Pellet Receptacle |
| Feces and urine tray | Feces and urine tray |
| Sound attenuating cubicle with (1) speaker - (1) circulation fan - (1) IR light | Sound attenuating cubicle with (1) speaker - (1) circulation fan - (1) IR light |
| Conduct Software: $1290 | Conduct Software: $1290 |
| Optional: TTL - Retractable levers or nose pokes - and more! | Optional: TTL - Retractable levers or nose pokes - and more! |
The Bussey-Saksida Touch Screen Operant Conditioning Chamber was developed by Dr. Jennifer Bussey and Dr. David Saksida who designed the chamber to address the limitations of traditional operant conditioning chambers, which they found were often less interactive and less adaptable for studying complex cognitive processes in animals.
The chamber can be used for multiple purposes, such as in psychological testing to assess visual discrimination, working memory, task-switching abilities, and other cognitive processes in animals. In pharmacological studies, the chamber enables researchers to investigate how various substances or treatments, such as drugs, affect behavior and cognitive abilities. In the field of comparative neuroscience, the device allows for the comparison of behavior across different species, offering insights into animal cognition and the neurological foundations of behavior.
The motivation behind the invention was to create a more flexible and sophisticated tool that could support a broader range of behavioral tasks, particularly those that require the animal to interact with visual stimuli. Traditional operant conditioning chambers were typically limited to simple tasks like pressing levers or responding to cues in the environment. In contrast, the touch screen chamber allows for more complex, task-specific interactions, such as visual discrimination, working memory tests, and cognitive flexibility exercises, which are crucial for studying higher-order cognitive functions.
By incorporating a touch screen interface, the chamber provides a versatile platform for testing a variety of cognitive processes, including attention, memory, and decision-making. It also facilitates more precise data collection, allowing researchers to monitor detailed behaviors and responses with greater accuracy. The invention was intended to push the boundaries of behavioral neuroscience and provide more accurate and nuanced insights into animal cognition, learning, and the effects of different substances or conditions on behavior.
The Bussey-Saksida Touch Screen Operant Conditioning Chamber was developed to study advanced cognitive and behavioral research in animals, facilitating a deeper understanding of the neural mechanisms underlying learning and memory.
 Sound attenutating chamber
Sound attenutating chamber
 Chamber
Chamber
 Pellet Dispenser
Pellet Dispenser
 Pellet dispenser x1
The pellet dispenser is controlled by Conduct software to release pellets via protocol or lever press
20 mg pellet dispenser is the default choice (tested with pellets from Bio-Serv).
45 mg pellet dispenser is also available
Lickometers also available
Pellet dispenser x1
The pellet dispenser is controlled by Conduct software to release pellets via protocol or lever press
20 mg pellet dispenser is the default choice (tested with pellets from Bio-Serv).
45 mg pellet dispenser is also available
Lickometers also available
 Touchsceen
Highly configurable touchscreen for multiple paradigms
Touchsceen
Highly configurable touchscreen for multiple paradigms
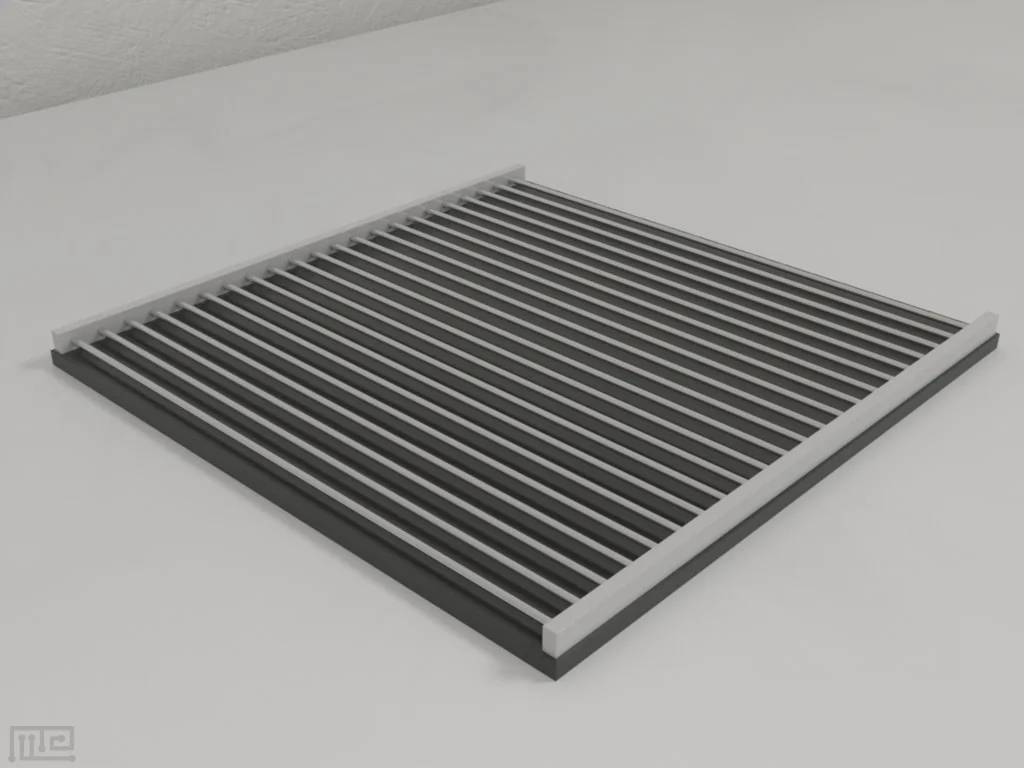 Shock grid (optional)
Specifcations: Constant current - 0.1 to 4.0 mA in 0.1 mA steps
Shock grid (optional)
Specifcations: Constant current - 0.1 to 4.0 mA in 0.1 mA steps
| Paradigm Name | Purpose | Application |
| Rodent Continuous Performance Task: Image (rCPT) | Examines Impulsivity - Attention - Cognitive flexibility and response inhibition | Alzheimer’s - OCD - ADHD - and Schizophrenia |
| Visual Discrimination & Reversal Learning | Tests learning ability - cognitive flexibility - and response inhibition | Executive function studies - schizophrenia models |
| Pairwise Discrimination Task | Assesses visual learning and object recognition | Alzheimer’s disease models; perceptual learning studies |
| Trial-Unique Nonmatching-to-Location (TUNL) Task | Evaluates spatial working memory and pattern separation | Hippocampal function studies; neurodegeneration models |
| Paired-Associate Learning (PAL) Task | Tests associative memory and object-location binding | Alzheimer’s disease models; aging studies |
| Delayed Matching-to-Sample (DMTS) Task | Assesses short-term memory and delay-dependent learning | Memory decay studies; cognitive aging research |
| Five-Choice Serial Reaction Time Task (5-CSRTT) | Measures attention; impulsivity; and response control | ADHD models; frontal cortex function studies |
| Progressive Ratio Task | Evaluates motivation and effort-based decision-making | Reward processing studies; depression models |
| Probabilistic Learning Task | Tests reinforcement learning and uncertainty processing | Dopamine-related decision-making studies; psychiatric disorder models |
| Object-Location Contingency Task | Examines spatial learning and memory | Hippocampal research; spatial cognition studies |
| Serial Reversal Learning Task | Tests cognitive flexibility and adaptability | Frontal cortex function research; neuropsychiatric disorder studies |
| Delay Discounting | Examines impulsivity; self-control; and decision-making | ADHD; substance use disorders; gambling addiction; and obesity. |
| Go/No-Go | Assesses inhibitory control; attention and executive function; and to model for impulsivity and impulse-control disorders | ADHD; obsessive–compulsive disorder (OCD); substance use disorders; frontal lobe damage; and impulsive aggression. |
Purpose: To familiarize the animal with the chamber environment.
Purpose: To teach the animal to interact with the touch screen.
Purpose: To train the animal on a specific cognitive or behavioral task.
Examples of Tasks:
Procedure:
Purpose: To record behavioral performance and analyze cognitive function.
Procedure:
Performance Metrics
Hit rate: proportion of correct touches to target stimuli.
False alarm rate: touches to non-target stimuli.
Reaction time: time between stimulus onset and touch.
d′ (d-prime): sensitivity index combining hit and false alarm rates.
Vigilance decrement: decline in performance over the session.
Stage 0 — Habituation (1–2 sessions)
Turn the sound-attenuating chamber house light on and deliver free rewards every 20–30 s. End the session when the subject collects ≥30 rewards/session.
Stage 1 — Autoshaping to touch (2–3 sessions)
Present the touch screen as a large white screen where any touch delivers a reward. The stimulus remains until the subject touches the screen (no limited hold yet).
Criterion: ≥60 touches/session until reward collection latency is stable.
Stage 2 — Must-touch target (2–5 sessions)
Present the target image only (e.g., white square) centrally and require the subject to touch the screen within a limited hold time (e.g. start 5 s → reduce to 2 s).
No response = omission; re-initiate after Inter-Trial Interval (ITI).
Criterion: ≥80% correct responses, omissions ≤20%.
Stage 3 — Introduce non-target (Go/No-Go, easy ratio) (3–6 sessions)
Randomized trials: 70% target (Go), 30% non-target (No-Go).
Responses:
Target + touch → reward (Hit); no touch → miss.
Non-target + no touch → correct rejection; touch → false alarm (timeout).
Use longer ITI after false alarms to discourage impulsivity (e.g., +2 s).
Criterion: d′ ≥ 1.5 (or Hit ≥70% with FA ≤30%) for two consecutive sessions.
Stage 4 — Balanced difficulty (50/50) (3–7 sessions)
50% target, 50% non-target; limited hold 1–2 s; ITI variable (mean 5 s).
Criterion: d′ ≥ 1.8 (typical), omissions ≤15%, stable across 2–3 sessions.
Stage 5 — Stability & parameter tightening (optional, 3–5 sessions)
Shorten limited hold (→1 s), increase ITI (3–7 s), shrink stimulus size or add distractors (e.g., brief houselight flicker).
Establish baseline across ≥3 stable days before experiments.
Stage 6 — Test sessions (experimental manipulations)
Keep parameters fixed from baseline.
If pharmacological interventions or lesions used in subjects, counterbalance the trial order and include washout days as needed.
ITI
Stimulus onset (target or non-target) + limited hold timer starts
Response window:
Target: touch = Hit (reward); no touch = Miss
Non-target: no touch = Correct Rejection; touch = False Alarm (timeout)
Outcome period (reward delivery or timeout)
Next ITI (longer after FA to discourage impulsivity)
Several studies have employed the Bussey-Saksida chamber to examine learning and memory processes. For instance, Talpos et al. (2010) used the chamber to assess visual discrimination and reversal learning in rodents. Their findings demonstrated that mice and rats exhibit similar cognitive flexibility impairments following prefrontal cortex lesions, highlighting the chamber’s utility for studying executive function.
Another study by Horner et al. (2013) examined the effects of hippocampal damage on paired-associate learning in rodents. Using the chamber’s paired-associate learning task, the researchers found that hippocampal lesions significantly impaired performance, reinforcing the role of this brain region in relational memory.
The touch screen chamber has been extensively used in research on Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD). Mar et al. (2013) utilized the chamber to test cognitive decline in a transgenic mouse model of Alzheimer’s disease, employing a delayed matching-to-sample task. They observed progressive deficits in working memory performance, aligning with human AD pathology.
Similarly, Graybeal et al. (2014) used the apparatus to study cognitive deficits in a Huntington’s disease model. The study found impairments in visual discrimination and attention tasks, supporting the use of the chamber in screening for cognitive dysfunction in neurodegenerative disorders.
In Parkinson’s research, Kamin et al. (2017) used the chamber to assess dopamine-related cognitive impairments in a rat model of PD. Their results suggested that dopamine depletion affects cognitive flexibility and motivation, reinforcing findings from human studies.
The chamber has also been instrumental in assessing attention, impulsivity, and decision-making. Romberg et al. (2013) used a five-choice serial reaction time task (5-CSRTT) to investigate attentional deficits in rodent models of attention-deficit hyperactivity disorder (ADHD). Their results indicated that ADHD-like rodents showed higher omission rates and impulsive responses, mirroring human ADHD symptoms.
In decision-making research, Bari et al. (2015) applied a progressive ratio task in the chamber to measure motivation and reward processing. They found that lesions in the orbitofrontal cortex led to altered decision-making, supporting theories that this brain region is crucial for cost-benefit analysis.
The Bussey-Saksida chamber has been widely used in drug testing and neuropharmacology. Heath et al. (2016) examined the effects of dopaminergic drugs on visual discrimination learning, finding that dopamine agonists improved performance, while antagonists impaired it.
Similarly, Granon et al. (2018) used the chamber to investigate the cognitive effects of cholinergic drugs in aged rats. Their results demonstrated that cholinesterase inhibitors enhanced performance in a delayed matching-to-sample task, mimicking the cognitive benefits seen in human Alzheimer’s patients.
One of the chamber’s most significant advantages is its translational potential, as it allows researchers to use the same behavioral paradigms across species. Nithianantharajah et al. (2015) compared rodents and non-human primates in a rule-learning task, demonstrating that both species exhibit similar patterns of cognitive flexibility. This cross-species approach strengthens the chamber’s role in bridging preclinical and clinical research.
Additionally, Bussey et al. (2012) emphasized the chamber’s utility in psychiatric disorder models, including schizophrenia and depression, noting that it allows researchers to test human-like cognitive impairments in animal models.
Bari, A., Theobald, D. E. H., Caprioli, D., Mar, A. C., Aidoo-Micah, A., Dalley, J. W., & Robbins, T. W. (2015). Dissociable effects of noradrenaline and dopamine lesions on probabilistic learning and reversal in the rat. Journal of Neuroscience, 35(3), 1636–1646.
Bussey, T. J., Saksida, L. M., & Rothblat, L. A. (2012). Dissecting cognitive function in rodent models of schizophrenia using the touchscreen testing method. Neuropharmacology, 62(3), 1351–1361.
Graybeal, C., Feyder, M., Schulman, E., Saksida, L. M., Bussey, T. J., & Brigman, J. L. (2014). The touchscreen operant platform for assessing executive function in mice. Nature Protocols, 9(11), 2738–2752.
Heath, C. J., Bussey, T. J., & Saksida, L. M. (2016). Motivational assessment in mice using the touchscreen operant platform. Frontiers in Behavioral Neuroscience, 10, 126.
Horner, A. E., Heath, C. J., Hvoslef-Eide, M., Kent, B. A., Kim, C. H., Bussey, T. J., & Saksida, L. M. (2013). The touchscreen operant platform for testing learning and memory in rats and mice. Nature Protocols, 8(10), 1961–1984.
Kamin, D., Sachs, B. D., & Sawa, A. (2017). The impact of dopamine depletion on decision-making and learning in Parkinson’s disease models. Behavioural Brain Research, 332, 128–139.
Mar, A. C., Horner, A. E., Nilsson, S. R., Kent, B. A., Kim, C. H., & Saksida, L. M. (2013). Progressive cognitive decline in a model of Alzheimer’s disease: A touchscreen-based assessment. Brain Research, 1527, 108–120.
Talpos, J. C., McTighe, S. M., Dias, R., Saksida, L. M., & Bussey, T. J. (2010). Trial-unique, delayed nonmatching-to-location (TUNL): A novel touchscreen-based automated task for assessing working memory in rats. Journal of Neuroscience Methods, 191(2), 199–209.
No questions yet for this product.
