$7,890.00 – $8,190.00Price range: $7,890.00 through $8,190.00
The rodent operant conditioning chamber is designed to evaluate reward-seeking and aversive behaviors. Our chamber is fully equipped for operant assessments that focus on voluntary, goal-directed actions influenced by past experiences.
Maze Engineers provides a standard operant chamber suitable for mice or rats, featuring a single pellet dispenser with receptacle, two LED lights, two retractable levers, an automated LED house light, a shock grid, and our Conduct software for setting up protocols. This chamber offers extensive customization options with a variety of add-ons and accessories (details below). For instance, you can incorporate an optical sensor for nose poking detection or swap out pellet dispensers for Lickometers.

MazeEngineers empowers preclinical neuroscience research with meticulously designed, customizable behavioral apparatuses. From manual classic mazes to fully automated smart systems, we provide the tools scientists need to capture high-quality, reproducible data for studies on learning, memory, anxiety, and depression.


Mouse |
Chamber size: 18cm (l) x 18cm (w) x 20cm (h) |
(2x) Retractable levers or nose pokes |
(2x) LED visual stimuli |
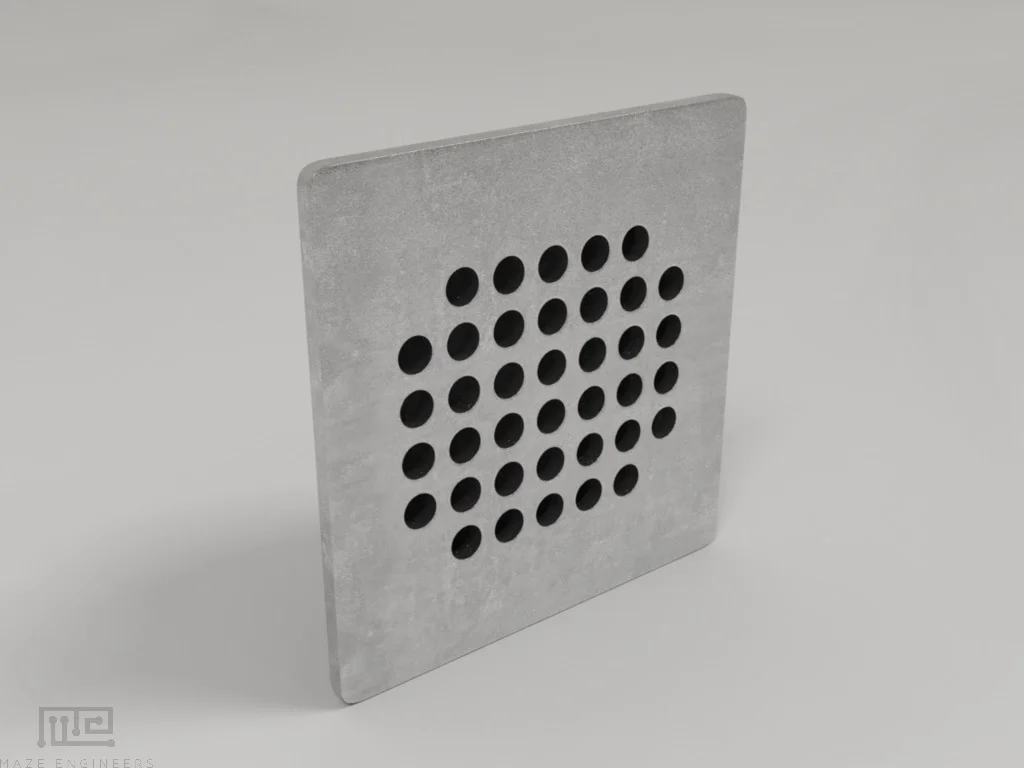
(1x) Shock Grid |
(1x) Pellet Dispenser |
(1x) Pellet Receptacle |
(1x) Tone speakers |
(1x) house light |
Feces and urine tray |
Sound attenuating cubicle with (1) speaker; (1) circulation fan; (1) IR light |
Conduct Software: $1490 |
Rat |
Chamber size: 26cm (l) x 26cm (w) x 25cm (h) |
(2x) Retractable levers or nose pokes |
(2x) LED visual stimuli |
(1x) Shock Grid |
(1x) Pellet Dispenser |
(1x) Pellet Receptacle |
(1x) Tone speakers |
(1x) house light |
Feces and urine tray |
Sound attenuating cubicle with (1) speaker; (1) circulation fan; (1) IR light |
Conduct Software: $1490 |
Operant behavior refers to a learned behavior in which animals link a particular event or stimulus to its outcome, leading them to modify their actions voluntarily. This connection forms after the animals experience consistent results from their behavior, which is evident in their altered likelihood of performing specific actions.
In essence, operant behavior develops as an adaptive mechanism when the consequences of an action become clear. Animals adapt their behavior to secure positive outcomes or steer clear of negative ones based on their previous encounters.
The concept of operant behavior was first introduced by American psychologist B.F. Skinner, who explored these patterns in his pioneering studies with laboratory animals. Operant behavior and conditioning help clarify the distinction between conscious and unconscious reactions, impacting various fields such as psychology, applied behavior analysis, and enhancing our insights into addiction, substance dependence, child development, and decision-making processes.
Within the operant chamber, subjects learn to avoid a lever or nose poke if it results in a shock, or they may learn to engage with these stimuli if they lead to a reward. Additional cues, such as lights and sounds, can also play a role in shaping their behavior.
Dimensions 60 x 55 x 65 cm (width x depth x height)
Multi-layer sound-proof insulation material that attenuates environment disturbance
Automated LED house light
Automated IR light
Automated air circulation fan
Camera mount
Pull-out floor shelf (optional)
Open slot for Optogenetic, cannuals, cables etc (optional)
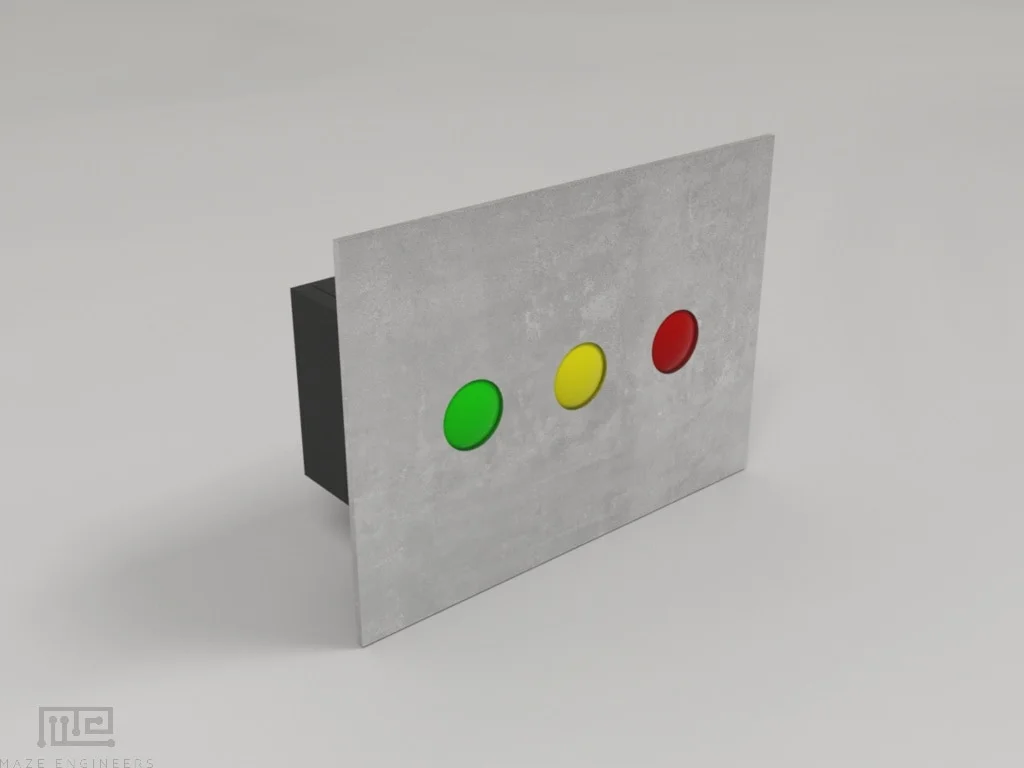
Each chamber is equipped with a cue light of configurable colors (white, red, green or blue)
The light on/off is controlled by Conduct software
Visible led light level 0-100, in steps of 5%
45 mg pellet dispenser is also available
Lickometers also available

Acrylic walls and base (Black or grey or blue or white)
Interior dimensions: Mouse: about 20x20x20 cm (LxWxH) to fit the grid of dimension 18×18 cm, Rat: about 30x30x30 cm (LxWxH) to fit the grid of dimension 27×27 cm, Custom dimensions available
Removable lid.
Removable feces catcher for easy cleaning
House light, two cue lights, speaker, one pellet dispenser, two levers (or nose pokes) with sensor detection.
Chamber construction supports optogenetic devices

Two retractable levers are made of stainless steel mounted on the interaction panel
Width: 1.6 cm for rats and 1 cm for mice
Nose pokes also available
A speaker per chamber
Frequency tone – 100-20,000Hz frequency; volume 1-130dB
Independent sound frequency and volume control per chamber
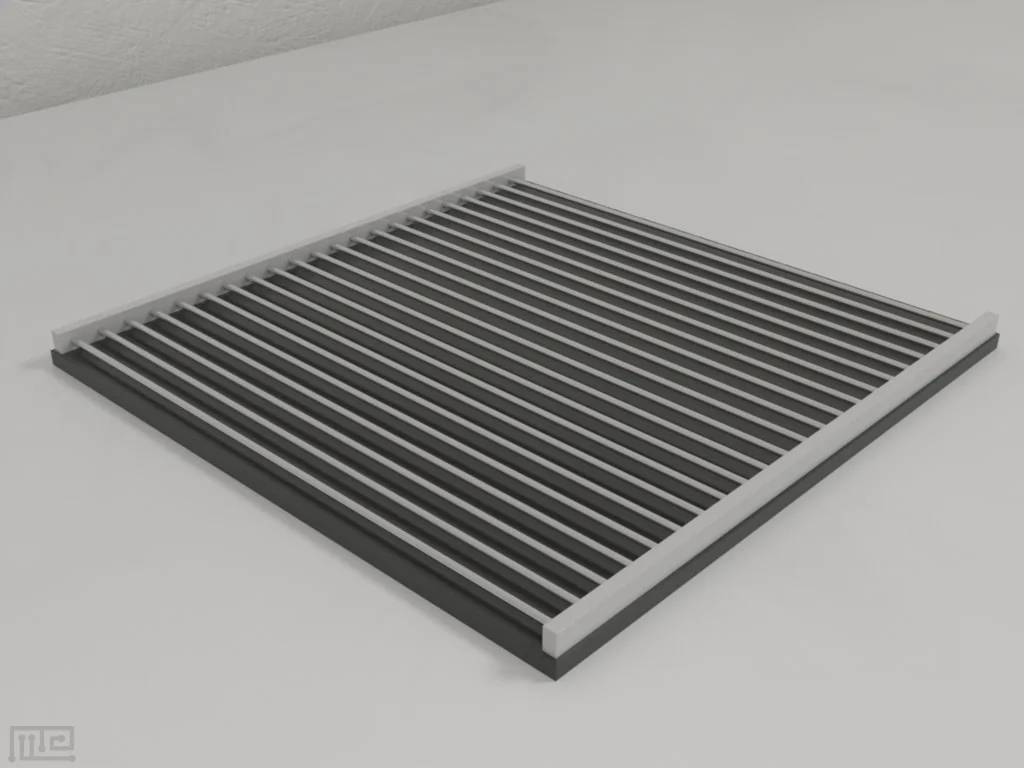
Shock current from 0.1 to 4.0 mA in 0.1 mA steps, programmable control
“Start/stop“ is controlled by Conduct software
Grid dimensions: Mouse: 18 x 18 cm with rod diameter 3mm and spacing 5 mm, Rat: 27 x 27 cm with rod diameter 5mm and spacing 10 cm

Take advantage of Neuralynx, Ethovision Integration, SMS and Email integration with the Conductor Science Software. No I/O Boxes Required
*Training schedules can be based on reinforcement or punishment with time or ratio-based protocols.
Borland et al. introduced a novel operant task designed to assess social reward and motivation in rodents. The authors aimed to develop a behavioral paradigm that could effectively evaluate the reinforcing properties of social interactions in rodents.
In the experiment, the authors utilized an operant conditioning chamber equipped with two response levers, each associated with a distinct social stimulus. The social stimuli included a conspecific (another rodent) and an inanimate object (a ball). The rodents were trained to respond on the lever associated with the social stimulus to obtain access to that stimulus.
The authors systematically manipulated different aspects of the task, such as the duration and intensity of social interactions, to investigate their effects on the rodents’ motivation to obtain social rewards. They also assessed the preference for social rewards over non-social rewards by comparing the response rates between the levers associated with the conspecific and the ball.
The results of the study demonstrated that the novel operant task effectively captured social reward-seeking behaviors in rodents. The rodents exhibited a higher motivation and preference for the lever associated with the conspecific compared to the lever associated with the inanimate object. This study highlights the relevance of understanding social impairments observed in psychiatric disorders and its potential for evaluating the effects of therapeutic interventions targeting social reward processing.
Overall, the study presents a valuable tool for investigating social reward and motivation in rodents using an operant conditioning paradigm, providing insights into the complex nature of social interactions and their importance in rodent behavior.
Dietary 2’-Fucosyllactose Enhances Operant Conditioning and Long-Term Potentiation via Gut-Brain Communication through the Vagus Nerve in Rodents
Vazquez et al. investigates the effects of dietary 2’-fucosyllactose (2-FL), a prebiotic compound found in human breast milk, on operant conditioning and synaptic plasticity in rodents. The authors aimed to explore the potential role of gut-brain communication mediated by the vagus nerve in these effects.
The experiment involved two groups of rodents: a control group and a group receiving dietary supplementation of 2-FL. The rodents were trained using an operant conditioning paradigm to associate a specific action with a reward. The researchers assessed the rodents’ learning and memory performance through operant response rates.
The results revealed that rodents receiving dietary 2-FL showed enhanced operant conditioning compared to the control group. They exhibited faster learning and increased response rates in acquiring the rewarded action. Additionally, the study investigated the underlying neural mechanisms by examining long-term potentiation (LTP) in the hippocampus, a key region involved in learning and memory.
The findings demonstrated that rodents receiving 2-FL exhibited enhanced LTP in the hippocampus, indicating improved synaptic plasticity associated with memory formation. The study further investigated the involvement of the vagus nerve in mediating these effects. By surgically manipulating the vagus nerve, the researchers demonstrated that blocking vagal transmission abolished the enhancements in operant conditioning and LTP induced by 2-FL.
Overall, the study highlights the beneficial effects of dietary 2-FL on operant conditioning and synaptic plasticity in rodents. It suggests that gut-brain communication through the vagus nerve plays a crucial role in mediating these effects. The findings contribute to our understanding of the impact of dietary components on cognitive processes and highlight the potential importance of gut-brain interactions in learning and memory.
Bardo, M. T., Donohew, R. L., & Harrington, N. G. (1996). Psychobiology of novelty seeking and drug seeking behavior. Behavioural Brain Research, 77(1-2), 23-43.
Bari, A., & Robbins, T. W. (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Progress in Neurobiology, 108, 44-79.
Dalley, J. W., Everitt, B. J., & Robbins, T. W. (2011). Impulsivity, compulsivity, and top-down cognitive control. Neuron, 69(4), 680-694.
Richardson, N. R., & Roberts, D. C. (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of Neuroscience Methods, 66(1), 1-11.
Skinner, B. F. (1938). The behavior of organisms. Appleton-Century.
Spielman, R.M., Dumper, K., Jenkins, W., et al. “Chapter 6.3 Operant Conditioning” Psychology, OpenStax, 2014, https://openstax.org/books/psychology/pages/6-3-operant-conditioning
Panlilio, L. V., & Goldberg, S. R. (2007). Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction Biology, 12(1), 1-22.
Pelloux, Y., & Everitt, B. J. (2016). Leveraging behavioral insights to optimize behavioral interventions for addiction. Current Opinion in Behavioral Sciences, 9, 74-80.
Vazquez, E., Barranco, A., Ramirez, M., Gruart, A., Delgado-Garcia, J. M., Jimenez, M. L., Buck, R., & Rueda, R. (2016). Dietary 2’-Fucosyllactose Enhances Operant Conditioning and Long-Term Potentiation via Gut-Brain Communication through the Vagus Nerve in Rodents. PLOS ONE, 11(11), e0166070. https://doi.org/10.1371/journal.pone.0166070
| Material | Acrylic, Stainless steel |
|---|---|
| Color | Black, Grey, Blue, White |
| Dimensions | 60 cm x 55 cm x 65 cm |
| chamber_interior_dimensions_mouse | 20x20x20 cm (LxWxH) |
| chamber_interior_dimensions_rat | 30x30x30 cm (LxWxH) |
| shock_grid_dimensions_mouse | 18 x 18 cm |
| shock_grid_dimensions_rat | 27 x 27 cm |
| shock_grid_rod_diameter_mouse | 3mm |
| shock_grid_rod_diameter_rat | 5mm |
| shock_grid_spacing_mouse | 5 mm |
| shock_grid_spacing_rat | 10 cm |
| shock_current_range | 0.1 to 4.0 mA in 0.1 mA steps |
| pellet_dispenser_default | 20 mg |
| pellet_dispenser_alternative | 45 mg |
| lever_width_rats | 1.6 cm |
| lever_width_mice | 1 cm |
| speaker_frequency_range | 100-20,000Hz |
| speaker_volume_range | 1-130dB |
| led_light_level_range | 0-100 in steps of 5% |
| cue_light_colors | white, red, green or blue |
| software_chamber_support | up to 16 chambers simultaneously |
| number_of_levers | 2 |
| number_of_cue_lights | 2 |
| number_of_pellet_dispensers | 1 |
| Species | Mouse, Rat |