Introduction
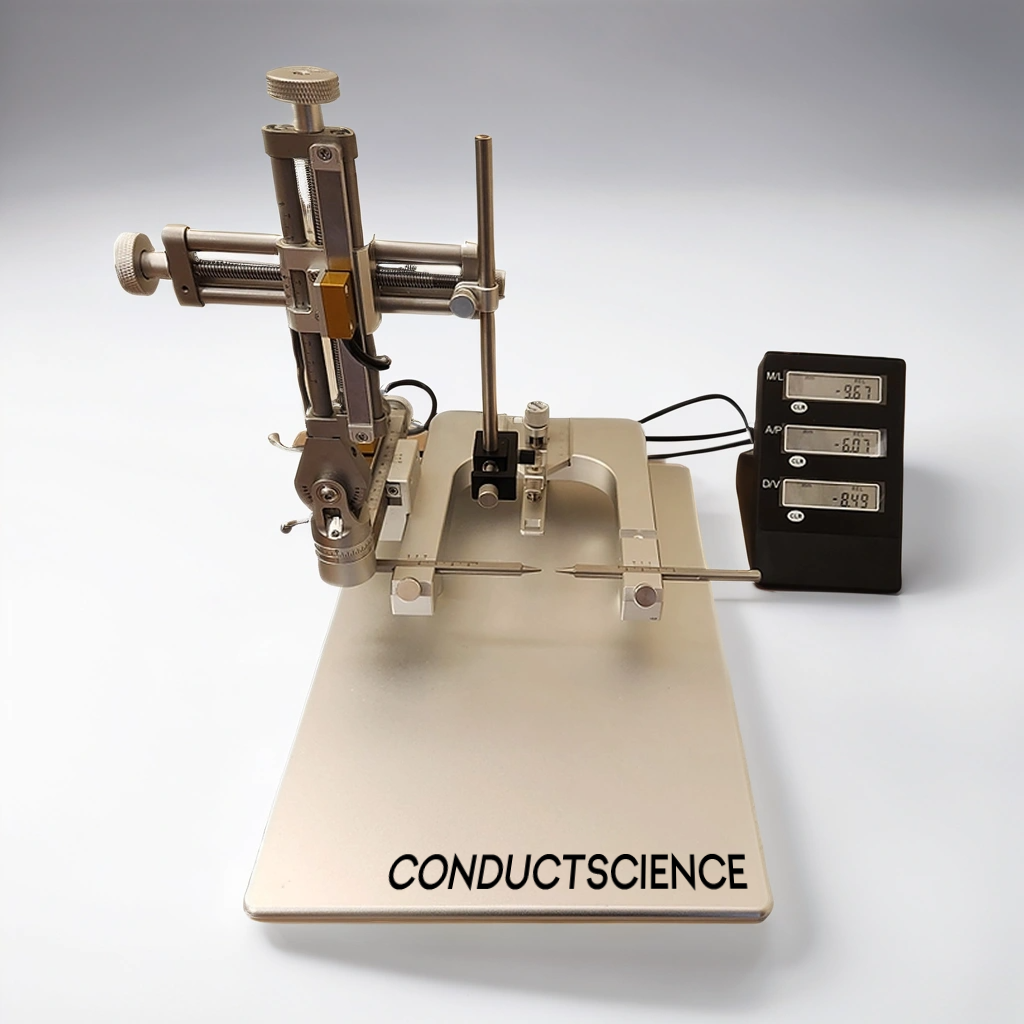
The Conduct Science Stereotaxic instrument uses landmarks on the surface of the animal’s skull (such as the Bregma ) as basic reference points and determines the position of a neural structure (brain region) through the directional movement of a three-dimensional manipulator arm. The device can then be used to perform injections, stimulations, impairment, guided positioning and other operations on the specific brain region . This device can also be used in conjunction with optogenetics, two-photon, endogenous brain imaging, fiber optic recording, electrophysiology and other systems.
Apparatus and Equipment
The Conduct Science Stereotaxic frame utilizes anatomical landmarks on the surface of the animal's skull, such as the bregma, lambda, sagittal suture, external auditory meatus, and tooth bar, to determine the X/Y/Z three-dimensional coordinates of functional brain areas beneath the cortex. The target region can then be reached through adjustment of X/Y/X axis using the main and vernier scales.
Triangular guide rails allow for quick positioning with universal contact points to facilitate lateral or longitudinal movement. The operating arms can be rotated at a certain angle from the bracket to facilitate the fixation of experimental animals.
The frame has been designed for optimum long-term use by replacing traditional metal-to-metal “hard grinding” transmission of the operating arm with a combination of screw rod and rolling mechanisms to maintain the stability and accuracy of the coordinate axis over time.
Molded transmission screws and thread sleeves and the use of connecting joints (rather than multiple joint connections) ensure stability and uniformity to reduce error in use.
Models
| Model
|
Species |
Specification |
| CS-STS-SM100D |
Mouse |
Single Arm |
| CS-STS-SM200D |
Mouse |
Double Arm |
| CS-STS-SR100D |
Rat |
Single Arm |
| CS-STS-SR200D |
Rat |
Double Arm |
Specifications
| Specification |
Result |
| Operating arm axis movement |
X, Y ,Z |
| Operating arm movement |
360 degrees; Swinging Range: 180 degrees |
| Reading accuracy |
Manual model 0.1MM (100um), Digital Model 0.01MM (10um) |
| Counting accuracy |
±0.01mm |
| Vernier Propeller Accuracy |
±0.01mm |
| Three dimensional propeller stroke |
80mm |
| Reading method |
Main and Vernier scales |
| Movement Range |
80mm |
| Positioning |
One click to default (bregma) |
| Dimensions |
350 × 250 × 340mm (L x W x H) |
| Power Supply |
1.5V DC power supply, no electronic interference, suitable for electrophysiological experimental environments |
| Composition |
Metal alloy |
Accessories
| No. |
Adaptor for Traumatic Brain Injury
(TBI) |
Model |
| 1 |
Rotational Mouse Adaptor, 60°Ear Bars |
CS-RMA-68065 |
| 2 |
Rotational Rat Adaptor, 18°Ear Bars |
CS-RMA-68063 |
| 3 |
Optional Precise Rotational Adapter For Mouse |
CS-PRMA-69100-03 |
| 4 |
Optional Precise Rotational Adapter For Rat |
CS-PRMA-69100-04 |
Technical Features
1
The three-dimensional operating arm is designed with a concentric directional transmission mechanism to maintain the consistency and accuracy of the transmission direction.
2
The operating arm replaces the traditional metal-to-metal “hard grinding” transmission (which wears fast) and adopts the combination of screw rod and rolling mechanism to maintain the stability and accuracy of the coordinate axis over time.
3
Key components such as transmission screws and thread sleeves are molded to ensure stability and uniformity when in use.
4
The use of connecting joints greatly reduces errors caused by multiple joint connections and improves stability.
5
The screw drive fixes the head, front teeth, nose bridge, and ear canal, which makes the adjustment smooth and precise, reducing the tedious operation steps of head fixation.
Applications
1. Brain Mapping
2. Lesion studies
3. Drug delivery
4. Electrophysiology
5. Gene therapy and viral vector delivery
6. Device implantation
7. Behavioural studies
8. Regenerative medicine
9. Traumatic Brain Injury (TBI) and Spinal Cord Injury (SCI) (see our
impactor line)
10. Neuroplasticity Studies
11. Pain Research
12. Neurodegenerative Disease Models
13. Pharmacological Testing
Procedure
Materials Needed:
- Stereotaxic instrument
- Anesthesia (e.g., isoflurane or injectable anesthetic)
- Subject
- Surgical instruments (scalpel, forceps, scissors)
- Sterile saline or PBS
- Antiseptic solution (e.g., iodine or chlorhexidine)
- Dental cement or sutures
- Heating pad
- Analgesics
- Personal protective equipment (PPE)
Procedure:
- Preparation:
- Ensure all surgical instruments and the stereotaxic apparatus are sterilized.
- Prepare the anesthetic setup (e.g., isoflurane vaporizer).
- Anesthesia:
- Anesthetize the subject using an appropriate method (e.g., isoflurane inhalation or injectable anesthetics like ketamine/xylazine).
- Verify the depth of anesthesia by checking the absence of reflexes (e.g., pedal reflex).
- Positioning:
- Place the subject on a heating pad to maintain body temperature and continue anesthesia.
- Move the adapter forward and backward to align the centerline of the left and right ear bars with the mouse's external auditory canal on the same line. Then, insert the left and right ear bars into the external auditory canal, adjusting the positions of the two ear bars to be symmetrical and at the same height, and tighten the screws to fix the ear bars. Furthermore, adjust the position of the mouse adapter's incisor clamp up and down so that its position is 3.3±0.4mm below the centerline connecting the left and right ear bars. Ensure the head is stable and level. Optionally, use a nose clamp and bite bar to further stabilize the head.
- Adjust the positions of the incisor clamp, nose bridge, and ear bars again, pressing firmly against the nose bridge. At this point, the head is fixed, the skull is horizontally leveled (note: this is a preliminary judgment criterion and further assessment is needed), and the nose is inside the anesthesia mask of the adapter, with breathing maintained by gas from the anesthesia machine
- Check that the subject's skull is successfully fixed: the nose is in the center, the tail is not slipping, visually confirm the brain is level, and pressing the subject's head from various directions (top, left, right, and side) does not result in movement.
- Surgical Site Preparation:
- Shave the the surgical site (usually the scalp).
- Clean the area with an antiseptic solution.
- Incision:
- Make a midline scalp incision using a scalpel to expose the skull.
- Retract the skin to clearly expose the bregma and lambda landmarks on the skull.
- Zero Point Localization: Secure a metal positioning needle in the stereotaxic instrument. Move the metal positioning needle above the sagittal suture, then move it forward and backward until the needle just touches the Bregma.
- Secondary Skull Plane Adjustment: Starting from the Bregma, for the first step, move the positioning needle backward along the sagittal line to approximately 2.5mm and 2mm to the left and right, respectively. Observe whether the positioning needle is at the same height from the skull surface at the Bregma and the 2.5mm position along the sagittal line, as well as at the 2mm positions on the left and right. For the second step, continue moving along the sagittal line to approximately 4.2mm and observe whether the positioning needle is at the same height from the skull surface at the Bregma and the 4.2mm position.
- Locating Stereotaxic Coordinates:
- Identify the bregma (anterior junction of coronal and sagittal sutures) and lambda (posterior junction) as reference points.
- Use these landmarks to determine the coordinates for the target brain region (e.g., striatum) according to a stereotaxic atlas.. Select appropriate coordinate points (e.g., AP +0.98mm, ML ±2.0mm, DV -3.0mm).
- Drilling:
- Mark the target coordinates on the skull.
- Drilling: Start moving the positioning needle from the Bregma. Locate a point 0.98mm anterior to the Bregma and 2.0mm lateral to the left or right side of the sagittal suture. Mark this point with a marker pen, which represents the position of the striatum in the X-Y plane.
- Select an appropriately sized drill bit slightly larger than the injection needle. Hold the skull drill manually or secure it to the stereotaxic instrument and drill at the marked point. Caution: Maintain a gentle touch and avoid exerting excessive force. When the drill bit breaks through, there will be a distinct sense of breakthrough. At this point, withdraw the skull drill.After drilling, carefully puncture the dura mater with the fine needle of a medical syringe to avoid damage. If there is bleeding during this process, use a very small medical cotton ball to absorb the blood.
- Implantation or Injection:
- Attach the appropriate instrument (e.g., needle, electrode, cannula) to the stereotaxic arm.
- Lower the instrument slowly and precisely to the desired depth in the brain or spinal cord.
- Perform the desired procedure (e.g., injection of a substance, implantation of an electrode). For example, for injections, set the injection parameters (syringe specifications [capacity, piston inner diameter], target injection volume, injection rate), and then start the injection (for example: striatum injection 1ul, rate approximately 0.5ul/min; virus injection 1ul, rate approximately 0.2ul/min). After injection, keep the needle in place for 5-10 minutes to allow for complete diffusion and absorption of the drug, then slowly retract the needle*.
- Sealing:
- If necessary, seal the hole in the skull with dental cement or suture the overlying skin.
- Ensure that the surgical site is clean and free of debris.
- Recovery:
- Remove the rodent from the stereotaxic apparatus and place it in a clean, warm recovery area.
- Monitor the rodent until it fully recovers from anesthesia.
- Administer post-operative analgesics as required.
- Post-Operative Care:
- Continue to monitor the rodent for signs of distress, infection, or pain.
- Provide any additional care as needed to ensure a smooth recovery.
*Before conducting the formal drug injection, it is necessary to perform a pre-experiment to verify the accuracy of the localization of the target brain area. It is recommended to use tracer dyes such as DiI (detectable after 1 week), fluorescent gold dye (detectable after 2 weeks), or red or black dyes (detectable after 1 week). Then, take samples for frozen sectioning, and compare them with some reference points in the brain atlas to determine the accuracy of brain area localization (selecting larger and clearer reference points, such as the hippocampus, lateral ventricle, and optic chiasm nucleus, etc.).
Note: When sectioning, approach the target nucleus from the outside, and ensure that the angle of brain sections is consistent with the brain atlas.