
The Calvin(-Benson-Bassham) (CBB) cycle, also named reductive pentose phosphate pathway or dark reactions, is a group of biochemical reactions in photoautotrophs.
These reactions form the light-independent stage of photosynthesis, where the energy converted from light is used to assimilate carbon dioxide from the atmosphere.
The fixed carbon molecules are incorporated into carbohydrates, which are consumed by the heterotrophs. The carbohydrate intermediates produced during the Calvin cycle can also be converted to precursors of proteins and lipids that also feed consumers in the food chain.
Photosynthesis in eukaryotic organisms such as cyanobacteria, green algae, and plants occurs in chloroplasts and can be divided into light-dependent and independent stages.
The light-dependent reactions begin when photoreceptors in the thylakoid membrane capture photons, energy from sunlight. The solar energy excites electrons in photoreceptors clustered into photosystems I and II (PSI and II).
The excited electrons go through the electron transport chain, reducing nicotinamide adenine dinucleotide phosphate (NADP+) into NADPH and oxidizing water into oxygen.
During the transfer of electrons, proton gradients are generated across the thylakoid membrane, leading to adenosine triphosphate (ATP) synthesis in the stroma; this process is called photophosphorylation.
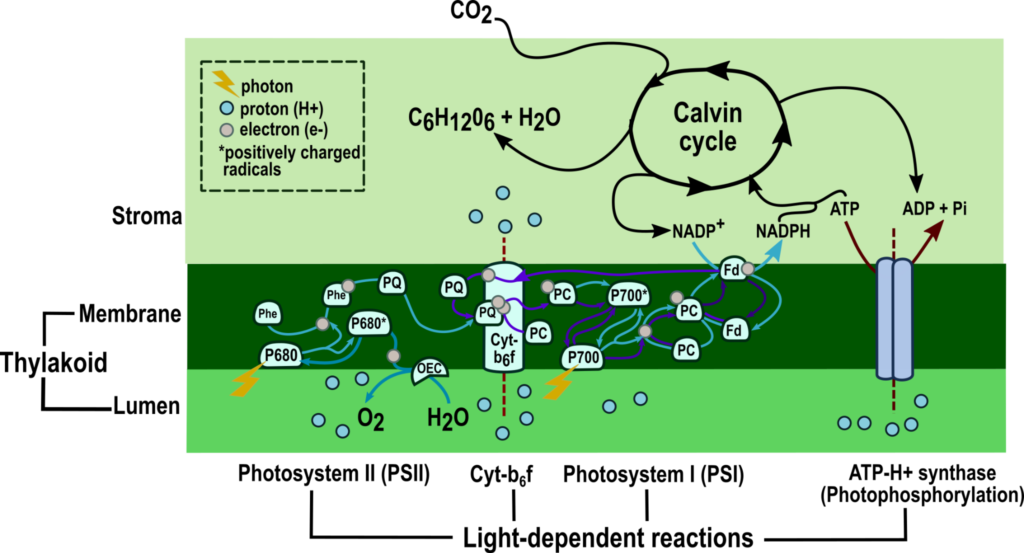
Figure 1: The two stages of photosynthesis: light-dependent reactions and the Calvin cycle.
(Source: Modified from Boyer, 2006 and Heldt, 2005).[1,2]
The second stage of photosynthesis occurs in a repeated set of biochemical reactions called the Calvin-Benson-Bassham or Calvin cycle, dark reactions, or the reductive pentose phosphate pathway (named after the cycle’s first substrate, a pentose phosphate).
The cycle starts by capturing atmospheric carbon dioxide (CO2), incorporating it into a five-carbon substrate, thus resulting in a six-carbon intermediate. Subsequently, ATP and NADPH generated during the light-dependent reactions are consumed to transform the six-carbon product into two molecules of a three-carbon phosphate sugar (triose phosphate).
A fraction of the triose phosphate is transported to the cytoplasm for carbohydrate synthesis – the final product of photosynthesis. Most of the triose phosphate molecules remain in the chloroplast so that they are used to regenerate the first substrate of the Calvin cycle – they leave the chloroplast only after they’ve been converted to DHAP.
NB: ADP and NADP+ from energy-consuming reactions are recycled to the light-dependent reactions so that they can participate in the electron transfer process.[1,2]

Figure 2: Overview of the Calvin cycle (Source: Mike Jones User: Adenosine, CC BY-SA 2.5 <https://creativecommons.org/licenses/by-sa/2.5>, via Wikimedia Commons).
Despite the name dark reactions, the Calvin cycle does not necessarily occur in the dark.
In fact, the Calvin cycle is coupled with water oxidation, the last electron transfer reaction in PSII that occurs after the capture of sunlight energy. Electron transfer helps generate NADPH and ATP, which are used in the Calvin cycle.
Therefore, the Calvin cycle is more likely to happen during daylight after the light-dependent stage has sufficiently produced NADPH and ATP.
The biochemical reactions in the Calvin cycle can be grouped into three phases based on their tasks:
Also known as the carboxylation phase, the first step in the Calvin cycle comprises only one irreversible reaction catalyzed by the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO). Read how enzymes are able to act as biocatalysts.
Here, atmospheric carbon dioxide (CO2) is fixed and incorporated into a five-carbon pentose phosphate, ribulose-1,5-bisphosphate (RuBP). The reaction produces a highly reactive six-carbon keto acid intermediate, 2-carboxy 3-keto arabinitol 1,5-bisphosphate.
The intermediate is hydrolyzed and split in half to yield two 3-phosphoglycerate (3PGA) molecules ultimately. Each is then subjected to successive reactions in the Calvin cycle and transformed into phosphate sugars.
RubisCo is the most abundant enzyme on Earth and the only one capable of fixing CO2 from the atmosphere. Thus, the rate of carbon fixation is considerably slow, which is thought to be the reason behind its large quantity in photosynthetic tissue.
Hence, RubisCo-catalyzed carboxylation acts as both the rate-limiting and committed step of the Calvin cycle.[2,3]
Apart from CO2, RubisCo also captures atmospheric oxygen (O2) and assimilates it to RuBP during photorespiration.
Instead of two 3PGA molecules, RubisCo-catalyzed oxygen condensation produces one 3PGA and another two-carbon molecule, 2–phosphoglycolate (2PGC).
The 3PGA molecule produced from photorespiration can supply the Calvin cycle in a similar manner to those produced from carbon fixation. However, the two-carbon product, 2PGC, must be translocated and modified before re-entering the chloroplast and converted to 3PGA before the fixed oxygen molecule can participate in the Calvin cycle.[2]
Each 3PGA generated from carbon fixation undergoes successive reduction reactions so that it is transformed into a triose phosphate (C3H7O6P). The three-carbon sugar phosphate can serve as a precursor in the biosynthesis of carbohydrates and the regeneration of RuBP in the last phase of the CBB cycle.
The transformation of 3PGA to triose phosphate occurs in two steps:
This first step in 3PGA transformation is catalyzed by the enzyme 3-phosphoglycerate kinase. The reaction dephosphorylates ATP and produces 1,3-bisphosphoglycerate (BPG), ADP and inorganic phosphate.
This second step in 3PGA transformation is catalyzed by NADP-glyceraldehyde 3-phosphate dehydrogenase. The carboxylic acid phospho-anhydride portion of the BPG reacts with the thiol group in the active center of the enzyme, resulting in the formation of a thioester bond. Subsequently, the thioester bond is hydrolyzed to form the product glyceraldehyde 3-phosphate (G3P).
Thioester bond hydrolysis requires a substantial amount of energy and is coupled with the oxidation of NADPH to NAPD+. Thus, the generation of G3P from BPG reduction is irreversible.
Thus, the reduction phase of the cycle can be summarized into:[2]
3PGA + ATP + NADPH —> Triose phosphate (G3P ⇌ DHAP)
Combined with the carbon fixation, both stages can be summarized into:
RuBP + CO2 + ATP + NADPH —> Triose phosphate (G3P ⇌ DHAP)
G3P is the first synthesized triose phosphate and it is converted to its isomer by the enzyme triose phosphate isomerase into dihydroxyacetone phosphate (DHAP). Both G3P and DHAP contain three-carbon atoms, and DHAP is more favorable in the equilibrium than G3P.[2]
One-sixth of the synthesized triose phosphate (G3P and DHAP) is used as precursors in the biosynthesis of sugar and starch.
When two triose phosphate molecules are available, they condense, forming a six-carbon molecule, fructose-1,6-bisphosphate (FBP). It is subsequently hydrolyzed into fructose-6-phosphate, an isomer of glucose-6-phosphate that serves as one of the precursors in sugar, starch, and cellulose biosynthesis.[2]
Generally, starch is synthesized from triose phosphate that remains in the stroma during the day. Sugar and cellulose are synthesized after triose phosphate is transported to the cytosol via a specific transporter.[3]
In addition to sugar and starch, G3P and DHAP can be converted into amino acids and fatty acids, which are building blocks for proteins and lipids.
The majority of the triose phosphate remains in the stroma and undergoes several chemical rearrangement reactions. At the end of the regeneration phase, the carbon-fixing RuBP is regenerated and available to restart the cycle.
In this phase, the reactions consist of a series of aldol condensation, dephosphorylation, and transketolase reactions that lead to the generation of a five-carbon sugar-phosphate – ribulose-5-phosphate (Ru5P).
The steps include:[2,3]
The three-carbon triose phosphate is transformed into six-carbon fructose-6-phosphate (F6P) in two steps:
In this step, F6B can exit the CBB cycle to supply other metabolic pathways as per cellular needs.
F6B which remains in the CBB cycle participates in several transfers of carbon atoms so that it is transformed into a five-carbon pentose phosphate molecule, ribulose-5-phosphate (Ru5P).
The enzymes transketolase or aldolase can catalyze the transfer of carbon atoms. Transketolase moves the carbon atoms from the ketone portion of the donor molecule and adds them to the aldehyde group of the accepting molecules. Aldolase catalyzes the condensation between ketone and aldehyde molecules.
The following are the metabolites and the number of their carbon atoms generated from transketolase-catalyzed carbon transfer:
Erythrose-4-phosphate (E4P) is a four-carbon metabolite resulting from the removal of two carbon atoms from F6B, facilitated by the enzyme transketolase.
Xylulose-5-phosphate (X5P) is a five-carbon metabolite generated from two transketolase-dependent transfers.
The first X5P is synthesized from the transfer of two carbon atoms from F6B to the three-carbon triose phosphate, G3P.
Later on, another X5P is generated in a similar manner. However, the two carbon atoms are from the ketone group of a seven-carbon metabolite, sedoheptulose-7-phosphate (S7P).
X5P is a Ru5P epimer and can be converted to Ru5P by the enzyme ribulose phosphate epimerase.
Sedoheptulose-1,7-bisphosphate (SBP) and sedoheptulose-7-phosphate (S7P) are seven-carbon metabolites in the light-independent stage of photosynthesis.
SBP is generated from the transfer of DHAP, the three-carbon metabolite, to the four-carbon metabolite, E4P, which is catalyzed by transaldolase.
S7P is subsequently generated from the irreversible dephosphorylation of SBP, catalyzed by the enzyme sedoheptulose-1,7-bisphosphatase.
Ribose-5-phosphate (R5P) is a five-carbon metabolite that is the residual from the transfer of two carbon atoms from S7P to G3P to form X5P.
R5P is a Ru5P isomer and can be converted to Ru5P by the enzyme ribose phosphate isomerase.
Thus, the processes in the generation of Ru5P can be summarized into:[2]
FBP + 2G3P + DHAP + 2H2O —> 2X5P + R5P + 2Pi
The two water molecules are used in the hydrolysis of FBP into F6P and SBP into S7P, which also produces two inorganic phosphates. X5P and R5P are enzymatically converted into Ru5P.
Thus, three Ru5P (3×5 carbon atoms) are generated from five molecules of triose phosphate (5×3 carbon atoms), two of which are condensed into FBP in the previous step of the regeneration phase.
In the last step of the regeneration phase, Ru5P is irreversibly phosphorylated by the enzyme ribulose phosphate kinase. The reaction consumes ATP and transforms Ru5P into ribulose-1,5-bisphosphate (RuBP).
RuBP synthesized at the end of the regeneration phase replenishes the RuBP in CO2 fixation. If ATP and NADPH are available, the regenerated RuBP will be consumed in the next round of the cycle.
The regeneration phase can be summarized into the following equation:[3]
5 Triose phosphate + 3ATP + 2H2O —> 3RuBP + 3ADP + 3Pi
Based on the summarized equation, three RuBP molecules are regenerated from five molecules of triose phosphate, which, based on the previous stages are from three rounds of CO2 fixation.
Rubisco-catalyzed CO2 fixation and assimilation into RuBP results in two 3PGA. Each consumes one ATP and NADPH and transforms into a triose phosphate (C3H7O6P) molecule. One-sixth of the triose phosphates produced by the cycle is used in carbohydrate synthesis pathways, while the rest remains and enters the regeneration phase.
Thus, the overall Calvin cycle can be summarized into:[3]
3CO2 + 5H20 + 9ATP + 6NADPH —> C3H7O6P + 9ADP + 9Pi + 6NADP+
Since ADP, inorganic phosphates, and NADP+ eventually resupply the light reactions, it can be concluded that the many biochemical reactions in the dark reaction contribute only one product to photosynthesis, a three-carbon triose phosphate, glyceraldehyde 3-phosphate (G3P). Two or more of the G3P molecules are subsequently used to synthesize starch and sugar.
The Calvin cycle starts with RubisCo-fixation of atmospheric CO2 and assimilation into a five-carbon ribulose-1,6-bisphosphate (RuBP), followed by a reduction phase, and the regeneration of its carbon-accepting substrate, RuBP.
Along the way, a fraction of the three-carbon product, glyceraldehyde 3-phosphate (G3P), leaves the cycle and serves as precursors in the biosynthesis of sugar and starch.
It marks the second stage of photosynthesis when ATP and NADPH produced during the first stage are consumed to set the stage for carbohydrate synthesis. And of course, it also enables photosynthesis to provide food and raw materials to heterotrophs in the ecosystem.
