
Electrophysiology refers to studying the electrical properties of biological cells and tissues. Thus, brain or neuronal electrophysiology pertains to a branch of electrophysiology that deals with measuring electric current or voltage changes in one neuronal cell, a brain slice, or the whole brain.
The methodology was first introduced by Yamamoto and Mellwain.[1] Now, it’s a major technical strategy to learn, understand, and get more insight into the physiology and pharmacology of the central nervous system (CNS).[1]
The central role of neurons can be categorized into the motor, sensory, and neurosecretory functions.[2] However, the electrophysiology of these structures is the one responsible for the generation of the action potentials. And these action potentials generated by different neurons show the extrinsic inputs from neurotransmitters and modulators and the integration of intrinsic properties of the membrane.[2]
Brain slice provides insights into the effect of such factors on the electrophysiological behaviors of the neurons of a specific part of the brain. The brain’s hippocampus is usually studied using the brain slice or sectioned brain region. However, it also has the potential to highlight the properties and function of each neuron of the CNS.
Furthermore, the electrophysiological studies of the brain slice play a major role in studying anesthetics and other drugs and understanding their neurophysiological responses.
This article discusses the techniques and applications of brain slice electrophysiology, and also briefly highlights how brain slice is prepared.
Related Read: Cardiac Electrophysiology: How to Setup a Cardiac Electrophysiology Lab
Different types of brain slices are prepared based on the goal of electrophysiology studies.[2] To create uniform slices without the need for freezing or embedding, tissue choppers are preferably used in labs for brain slice preparations. There are mainly three brain slice preparations followed in labs in the context of the question of interest.
They are ~150-350 μm thick, allowing the visualization of neurons under a compound microscope at high magnification for patch-clamp recordings.[2] However, the neurons at the surface aren’t of the best quality and do not provide accurate data or input in the functioning of the neurons. The technique is usually best to study neurons’ intrinsic membrane properties in whole-cell or isolated membrane patches.[2]
Recent microscopic advancements, such as the development of infrared microscopy have allowed the examination of the deeper cells and neurons of the brain slice, thus broadening the applications of thin slice preparations.[2]
They are generally ~500 μm thick and frequently used in brain slice preparations.[2] Because they maintain the local connections between neurons, they are used to examine intrinsic membrane properties, study local synaptic circuits, and drug effects in relatively intact cells.[2]
Additionally, it also has applications in the analysis of network activity, determination of the input pathways through electrical stimulations, and study of the pharmacological manipulations of the brain.[2]
The individual neurons manifest the membrane characteristics of cells in the brain slice. Thus, they are best used to study intrinsic cellular properties.[2]
Isolated neurons have major applications in workflows like single-channel recordings from isolated patches, calcium imaging, whole-cell patch clamp, and intracellular recordings.[2]
Earlier, brain electrophysiology was studied by attaching electrodes to the scalp and measuring brain potential in large areas of the brain. However, in the 20th century, with the discovery of microelectrodes, many electrophysiological recording techniques were developed by scientists, such as the patch-clamp technique, extracellular and intracellular recording, and optical imaging techniques.[3]
In this method, electrodes are inserted into the extracellular fluid, near the cell of interest, to measure the electrical activity being received from neighboring cells or neurons.[4] Here, impedance is determined by the capacitive properties of the electrode-cerebrospinal fluid interface, which are related to electrode material and tip size.
Generally, microelectrodes made of tungsten, gold, insulated steel, or platinum microwires are used to measure the impedance of many neurons simultaneously.[5]
The extracellular recording is further divided into four main groups:[6]
It’s used to determine the general activity of a single neuron in a specific brain region.[2] The limitation of the technique is that it only allows the study of neurons that generate an action potential. Thus, the cells in the silent state or not firing action potential can not be studied using the technique.[2]
The recording is used to study a population of neurons.[2] It allows for the simultaneous assessment of the activities of a group of neurons together (also called unit or synchronous activity). This type of recording is especially useful to describe the excitatory and inhibitory influences on a population of neurons.[2]
It’s done to measure and record the activities of peripheral nerves, such as the bundle of axons.[6] It’s either performed by using telemetry products to record sympathetic nerve activity or by placing a silver wire electrode near the nerve of interest.[6]
Field potentials are recorded from deep within the brain’s cortical tissue. It measures the field potential generated by many cells rather than an individual neuron.[6] It’s of different types, which include electroencephalography (EEG) and electromyographic (EMG).[6] The procedure involved in field potential recording on the skin is either invasive or non-invasive.
In this method, a glass microelectrode is placed against the cell membrane, which leads to the formation of a bond (or high resistance seal) between them known as gigaseal.[7] The contact between the glass pipette and membrane is confirmed by a sudden increase in the resistance.
To prevent the contamination of debris at the pipette tip, when moving inside the cell, a positive pressure is applied. Furthermore, to establish the bond/seal between the pipette and the membrane, voltage pulses or current is applied across the electrode.[7]
The technique can monitor currents passing through ion channels in the membrane within the gigaseal area, which has major applications in studying the basic neuronal membrane properties and synaptic transmission.[7]
When there’s a requirement for the transition of gigaseal to whole seal recording, the seal patch of the membrane is ruptured by applying more suction to the glass pipette.[7]
It monitors the changes in voltage-dependent currents. The basic clamp sets the membrane at the desired voltage.[8] Then, the desired current is added, which allows the measurement of membrane potential across the cell membrane.
Voltage clamp recording is of two types: two-electrode voltage clamp and single electrode voltage clamp. It is suitable to study the current-voltage relationships of membrane channels.[8]
The limitation of performing the technique using the sharp electrode is electrode resistance. Additionally, it is not feasible to clamp the entire cell, which makes it difficult to measure their membrane potential using this technique.[8]
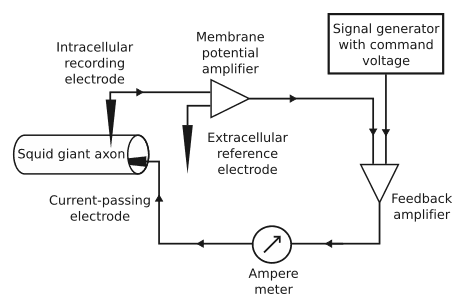
Figure: A schematic diagram of the negative feedback of the voltage clamp.[8]
The intracellular recording is the most traditional approach for recording changes in an individual neuron.[7] Additionally, it can be used to measure the action potentials, postsynaptic potentials, and after potentials of the neurons of interest.
The technique involves the use of a glass pipette with a diameter of less than 20-micrometer. An electrical connection is made between an electronic amplifier and a display through the use of sharp intracellular electrodes that are filled with a conductive saline solution. And through the saline solution in the pipette, an electronic amplifier is connected to the inside of the cell.[7]
This allows researchers to measure the electrical activity within the cell and obtain maximum information related to neuronal or brain activities.[7] However, as the cell is damaged during the penetration of the pipette, it can’t be permanently implanted for neural prostheses.[7]
Brain slice or neuronal electrophysiology is a technique used in neuropathology and similar labs to study and assess the electrical activity of an individual or a population of neurons. The measured membrane potential provides insight into the physiological condition of the brain, its responses to certain stimuli, and the activities of different brain regions.
Brain slice electrophysiology also has a major application in studying the responses of intact brain cells toward drugs. However, the effectiveness of the study depends on the brain slice preparations and electrophysiological approach used to study the actions or membrane potentials of the brain slices.
A range of electrophysiology techniques used in labs for such studies includes patch-clamp technique, voltage recordings, extracellular and intracellular recordings, and current-clamp recording.
If you need a slice workstation to help evaluate tissue slices, then check out our Semi-Automated Slice Workstation.
