
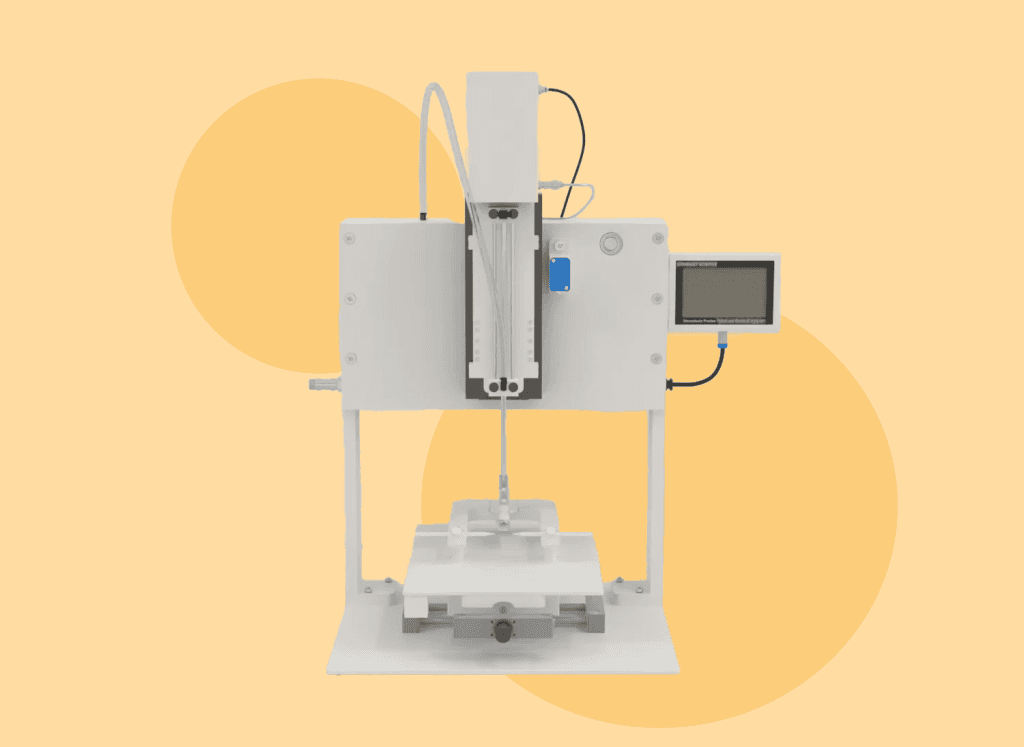
The stereotaxic impactor is designed to induce traumatic brain injury (TBI) through controlled cortical impact modeling. This advanced equipment allows researchers to create highly reproducible neurotrauma models, ensuring precise impact direction and injury location.
It provides researchers with full control over the biomechanical parameters of brain injury, including velocity, depth, dwell time, and the force applied by the tip. ConductScience’s Stereotaxic Impactor also allows for customization of the tip size, geometry, and position to accommodate different species. Together, these features enable researchers to address multiple histopathological and functional questions related to brain injury, making it an indispensable tool in neurotrauma research.
Stereotaxic procedures are frequently used to eliminate, enhance, or modify the function of certain brain regions as part of neurological research, pharmacological evaluation or central nervous system (CNS) disease-related experiments. [1]
The apparatus is a versatile and indispensable tool in both preclinical and clinical research settings. Its applications span a wide range of fields within neuroscience and beyond, providing precise localization and manipulation of specific brain regions. Some key applications include:
Neuroscience Research:
Pharmacological Research:
Behavioral Studies:
Advanced Neuroscience Techniques:
Clinical Applications:
Developmental Studies:
Embryonic and Neonatal Research: The apparatus can be adapted for use with very small animal subjects, such as embryos or neonates, facilitating studies on brain development and the effects of early-life interventions. [14]
Traumatic brain injury is one of the leading causes of morbidity and mortality among young people around the world. [15]
Animal models are commonly used to study brain trauma and test new treatments. The Controlled Cortical Impact (CCI) model is the most popular and accepted method for researching the physical, mental, and behavioral problems that result from brain injury. CCI is favored because it allows precise control over how the brain tissue is damaged. [16]
Here’s a step by step guide for TBI induction:
The stereotaxic apparatus is an essential tool in neuroscience and biomedical research. It is crucial for brain injury research as it provides precise and controlled induction of TBI.
This tool allows researchers to replicate brain injuries with high accuracy in terms of impact location and severity, ensuring consistency across experiments. By using the stereotaxic impactor, scientists can study the physiological, histological, and behavioral effects of TBI in a reproducible manner.
This precision is essential for understanding the mechanisms of brain injuries and for developing effective treatments and therapies.



Dr Louise Corscadden acts as Conduct Science’s Director of Science and Development and Academic Technology Transfer. Her background is in genetics, microbiology, neuroscience, and climate chemistry.
