

The nitrogen cycle is a group of biogeochemical processes that harvest the element (nitrogen) from the Earth’s reservoirs, transform it into biologically active chemical forms so that organisms in the ecosystem can consume and reap benefits before returning the element to its sources.
The chemical transformation in each nitrogen cycle step redistributes nitrogen between the environment and organisms in the ecosystem. In doing so, organisms can use naturally available nitrogen while the nitrogen content in the global environment remains unchanged. Over time, the nitrogen cycle can gradually shape and change the structure and composition of the ecosystem.
Nonetheless, human activities in the past centuries have dramatically increased nitrogen content. Moreover, the non-natural nitrogen input disturbs the global nitrogen cycle and negatively impacts the global climate and Earth’s ecosystems.
Nitrogen (N) is one of the fundamental elements of life on Earth, constituting a functional group in biomolecules such as proteins, nucleic acids, and chlorophylls.
It exists in various chemical forms and redox states. In living organisms, nitrogen exists as organic nitrogen (R-NH3).
Most nitrogen on Earth, however, is from inorganic sources. Small fractions are sporadically distributed in various forms on the Earth’s surface, such as in soils, ocean floors, rocks, and sediments. Most inorganic nitrogen is in the form of an inert, colorless, odorless, and tasteless gas called dinitrogen (N2) found in the atmosphere.[1]
The nitrogen cycle allows organisms to consume and use nitrogen in the environment before returning it to Earth’s reservoirs.
It consists of a series of redox reactions that transform nitrogen into various chemical forms so that it is biologically available. Here, nitrogen is transferred from the environment to the organisms and moved from one location to another without increasing or reducing the size of the global nitrogen content.[1]
Hence, the amount of nitrogen in the nitrogen cycle at a specific location is viewed as a budget. The input refers to the acquisition and transformation of non-reactive nitrogen into a biologically available form, called fixed N.
The output refers to the nitrogen released to the environment. Between the input and output is the internal cycling of nitrogen, consisting of several reactions that take place in microorganisms, plants, and animals.[2,3]
The nitrogen cycle occurs when free oxygen is present (oxic condition) or when it is in short supply (anoxic condition).[1,3]
The nitrogen cycle comprises the following steps:[2]
N2 + 8H+ + 8e– —> 2NH3 + H2
In diazotrophs, nitrogenase complexes, consisting of enzymes nitrogenase and nitrogen-reductase, are used to fix and transform atmospheric nitrogen.
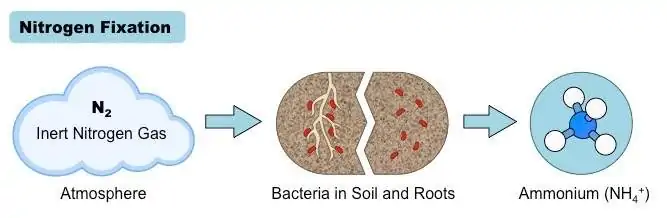
Immediately after fixation, the fixed nitrogen is assimilated by organisms such as bacteria, fungi, algae, and plants, transforming inorganic nitrogen into organic nitrogen (R-NH3) that the organisms can use in their anabolic processes.[1,4]
Plants and microorganisms can absorb nitrate (NO3–), ammonium (NH4+), and ammonia dissolved in water. Most are found in soils, ocean beds, or acquired from nitrogen fixers.[1,2]
In the case of nitrate, it is first reduced into nitrite (NO2–), which is further reduced to ammonium. The first reaction is catalyzed by the enzyme nitrate reductase and nitrite reductases, respectively. Ammonium is subsequently incorporated into amino acids as an amino group (NH3+).[4]
The nitrogen cycle’s central players in the nitrogen assimilation step are the enzymes glutamine synthetase (GS) and glutamate synthase, which is also named glutamine oxoglutarate aminotransferase (GOGAT).
GS catalyzes the incorporation of an amino group into glutamate, resulting in glutamine. GOGAT catalyzes the glutamine’s reversion to glutamate, a substrate for the synthesis of other amino acids, including ɑ-ketoglutarate, an intermediate in the Krebs cycle.[1,2]
Also known as mineralization, it converts organic nitrogen (R-NH3) into inorganic nitrogen such as ammonia or ammonium.[2]
Ammonification occurs when nitrogenous compounds in plant and fungal remains, animal corpses, and excretion decompose. It results in the breakdown of proteins, amino acids, and genetic materials, subsequently hydrolyzed by proteolytic enzymes.
Ammonification in terrestrial ecosystems is largely carried out by plants, fungi, and small animals. In marine ecosystems, it is facilitated by non-photosynthetic microorganisms. The resulting ammonia is used to assimilate nitrogen-containing compounds, increasing the biomass. It can also be oxidized to nitrate during nitrification.[1,2]
Under anoxic conditions, ammonium can be converted from nitrate or nitrite in dissimilative nitrate/nitrite reduction to ammonia (DNRA). It is present in many nitrogen-reducing bacteria and fungi in ocean sediments, ocean shores, human gastrointestinal tracts, and wastewater plants.[1,2]
In nitrification, ammonium is oxidized to nitrate (NO3–) in a series of redox reactions. It takes place at neutral to slightly alkaline pH in terrestrial and aquatic ecosystems.
The oxidation of ammonium to nitrate comprises two steps:[1,4]
2NH3 + 3O2 —> 2NO2– + 2H+ + 2H2O
NO2– + H2O —> NO3– + 2H+ + 2e–
The reduction of oxygen:
O2 + 4H+ + 4e– —> 2H2O
Both are summarized into the following redox:
2NO2– + O2 —> 2NO3–
Most aerobic microbes can perform only one step of nitrification. Only a specific group of microorganisms called Complete Ammonia Oxidizers (COMAMMOX) can carry out both nitrification steps.
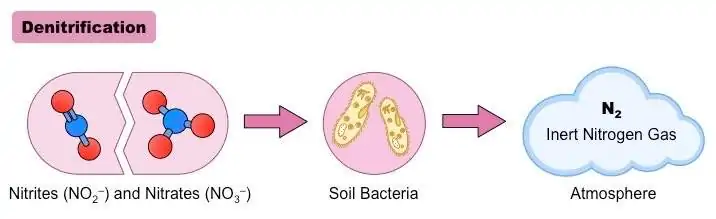
In this step, nitrate (NO3–) is reduced to dinitrogen (N2) and released into the atmosphere. It is the last step of the nitrogen cycle and is considered the output of the cycle.[1,4]
When the oxygen level is insufficient, nitrate is used as the terminal electron acceptor in denitrifiers’ cellular respiration. Subsequently, nitrate is sequentially reduced to dinitrogen (N2), illustrated as follows:
NO3– —> NO2– —> NO —> N2O —> N2
Complete reduction of nitrate involves several enzymes like nitrate reductase, nitrite reductase, nitrous oxide reductase, and nitric oxide reductase.[1]
An incomplete suite of nitrate reductase enzymes is the reason that many denitrifiers cannot fully reduce nitrate, leading to the release of the two intermediates, nitric oxide (NO) and nitrous oxide (N2O), into the atmosphere.
In addition to denitrification, anaerobic ammonium oxidation, or ANAMMOX, can produce dinitrogen gas (N2) that serves as the output of the nitrogen cycle.
Here, the oxidation of ammonium (NH4+) to N2 occurs in conjunction with the reduction of nitrite (NO2–), as illustrated in the summarized equation:
NH4+ + NO2– ⇌ N2 + 2H2O
ANAMMOX is carried out by autotrophic Planctomycetes species, which are found in marine sediments and wastewater plant sludge.[1,2]
Nitrogen is considered one of the limiting factors in primary production. Atmospheric nitrogen fixation, the subsequent transformation, and the internal cycling steps enable autotrophs to use nitrogen that may not be readily accessible in the environment.
In this process, the nitrogen cycle also allows the element to be redistributed to areas where nitrogen is limited. Plants, microorganisms, and animals that participate in one of the nitrogen cycle steps can mobilize the element from nitrogen-rich to nitrogen-limited areas.
For example, degraded soils are often first colonized by legumes and actinorhizal plants that form mutualistic symbiosis relationships with nitrogen-fixing bacteria.
In this symbiosis relationship, the bacteria fix nitrogen from the atmosphere and provide it to the plants. As a result, the plants can use the fixed N to produce food, which initiates subsequent biochemical reactions that increase the soil organic matter, restoring the soil in the process.[4] Read more about soil biology here.
Due to alterations in the structure and species composition of the ecosystems, the disparity in the nitrogen cycle steps can affect the global climate. How?
For example, nitrous oxide (N2O) produced from nitrification and incomplete denitrification is a greenhouse gas. The gas can be reduced to dinitrogen in the atmosphere, but excessive gas can interact with photons, transforming to nitric oxide, thus damaging the ozone layer. This reduces the ability of the Earth’s stratosphere to insulate heat while absorbing ultraviolet rays.[1,2]
Molecular mechanisms of ANAMMOX and the denitrification step of the nitrogen cycle are the basis for biotechnological solutions to wastewater.
Denitrification and ANAMMOX involve the microbial conversion of ammonium and nitrate to the chemically inert dinitrogen gas, which can be released to the environment without causing immediate harm.
For this reason, either process is applied to eliminate excess nitrogen from drinking water and develop new wastewater treatment systems, which have the potential to eradicate problems arising from acidification and eutrophication.[1]
The nitrogen cycle is a series of bio- and geo-chemical reactions that transform nitrogen and cycle it through the environment and organisms in the ecosystem. It enables organisms to acquire and use the element without depleting the global nitrogen content.
This cycle can gradually influence the life of organisms and the ecosystems they inhabit. Along the same line, human activities that overburden any nitrogen cycle steps can lead to environmental problems that devastate the ecosystems.
Nonetheless, some steps in the nitrogen cycle are applied to treat wastewater, which can be the key to addressing human-influenced environmental concerns.
