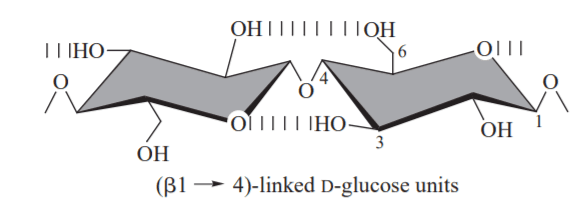
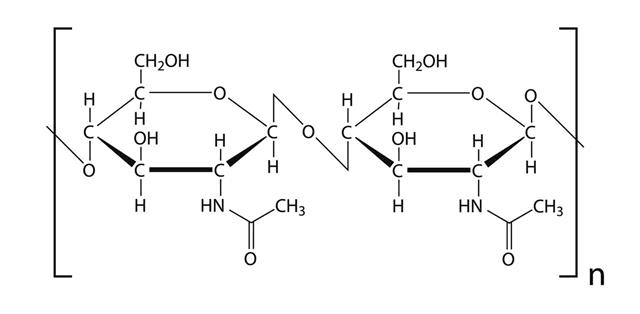
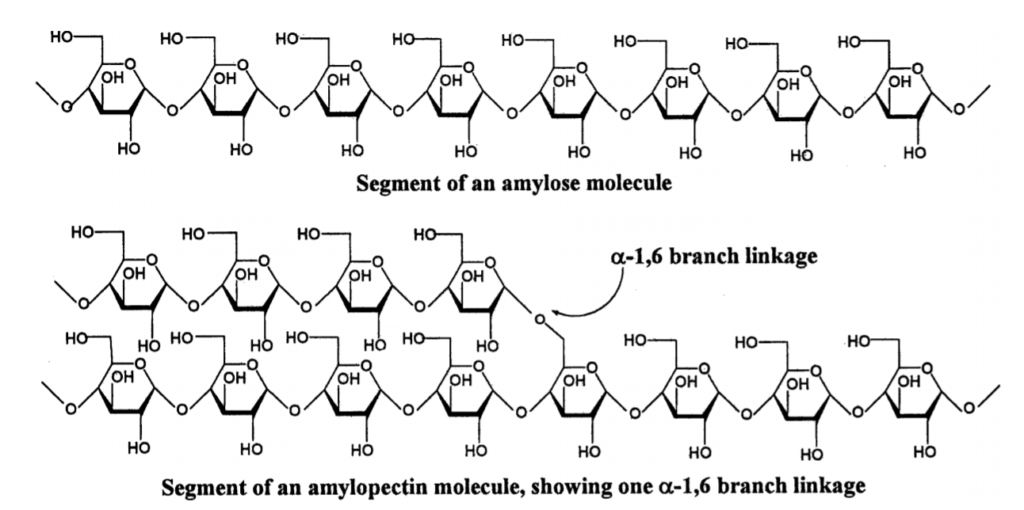
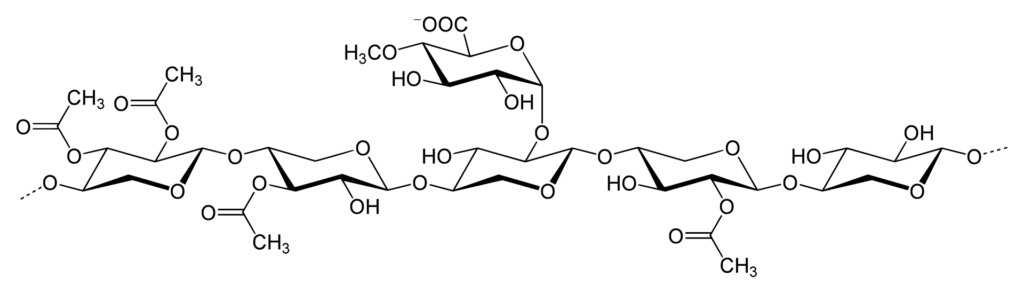
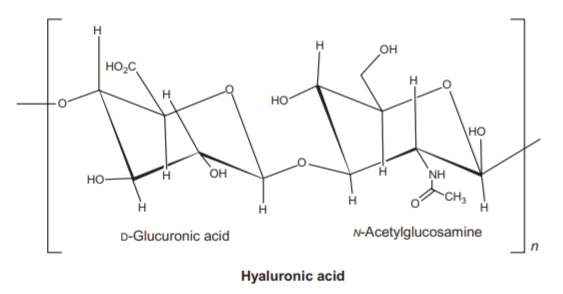
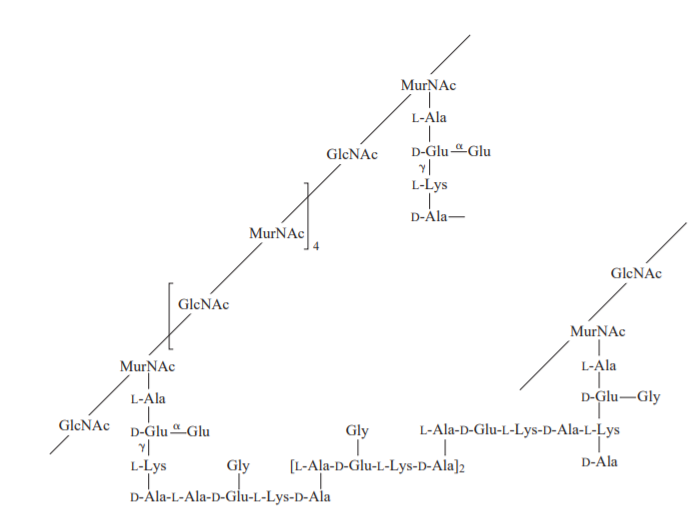
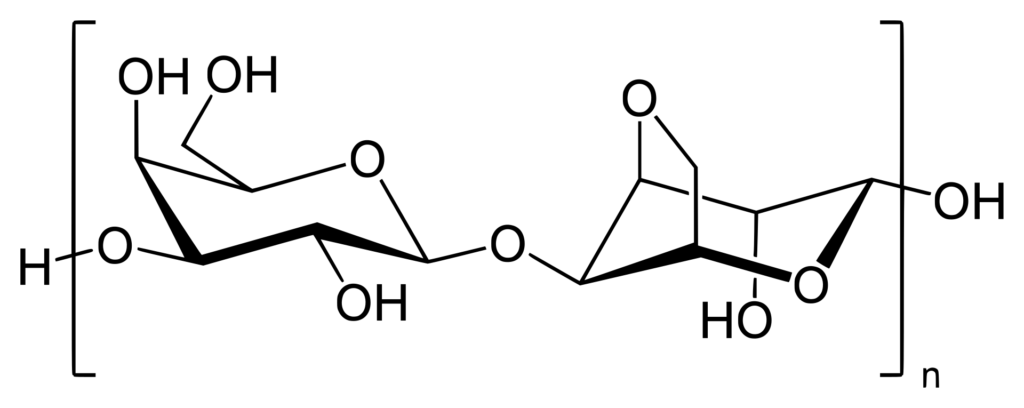
Polysaccharides are long-chain polymers of repeating units of sugars. They perform different life-supporting functions in different organisms. Some polysaccharides, because of their strong long polymer chain structure, elasticity, and rigidity, are used in several biomedical applications. Some of them, like agars and gellan gums, provide nutritional value and are used in the food industry.
The study of polysaccharides is still an ongoing research area. Many natural polysaccharides are being explored for their use as nanoformulation, microparticles, tablets, aerogels, hydrogels, and transdermal drug-delivery systems.
The natural physico-chemical properties of polysaccharides pose several challenges for their use in pharmaceuticals. However, scientists are working towards chemically modifying the physico-chemical properties of natural polysaccharides to develop novel semisynthetic natural polysaccharides that support their application in biomedical and pharmaceutical areas.