$5,490.00 – $7,990.00Price range: $5,490.00 through $7,990.00
The Maze Engineers automated single belt rodent treadmill utilizes ultra-quiet precision mechanical mechanisms to deliver the best possible treadmill on the market multiple features include easy variable sloping and acceleration, easy grip, Conductor software integration, rich data collection, and easy to protocolize treadmill for activity experiments. Please see the features section below for more information
Key Features include:
Single and multiple-lane treadmill models are available

MazeEngineers empowers preclinical neuroscience research with meticulously designed, customizable behavioral apparatuses. From manual classic mazes to fully automated smart systems, we provide the tools scientists need to capture high-quality, reproducible data for studies on learning, memory, anxiety, and depression.



Characteristic | Mouse | Rat |
|---|---|---|
Individual Lane Size | 17.32 x 2.36 x 5 inch (44 x 6 x 12.7 cm) | 17.32 x 4.72 x 5 inch (44 x 12 x 12.7 cm) |
Shock Grid Size | 4.72 x 2.36 inch (12x 6 cm) | 4.72 x4.72 inch (12 x 12 cm) |
Price for single lane | $5490 | $5990 |
Price for 5 lane | $6990 | $7990 |
The treadmill is a popular exercise system used to force-train rodents. The apparatus has a simple construction and provides the experimenter with the ability to vary the speeds and inclinations of the runway belt. Most treadmill systems utilize shock grids to motivate the animal to keep running. However, this can potentially result in pain stress influencing exercise training. Other methods of motivation such as a gentle tap or air-puffs may be used as a relatively less stressful alternative.
The treadmill is a simple tool to evaluate the effects of exercise and different-intensity training on not just physical health but also cognitive (Hwang et al., 2016) and mental health (Costa et al., 2012). Experimenters have the opportunity to observe the simultaneous performances of different treatment groups when using a multilane treadmill. The use of a treadmill is not limited to physical activity. The apparatus can also be used to investigate physical exhaustion (Dougherty, Springer, & Gershengorn. 2016) which can often be a symptom of diseases and disorders. Further, assessment of motor and locomotion function post-recovery from injuries can also be done using the treadmill (English, Wilhelm, & Sabatier, 2011).
The treadmill consists of a motorized runway and associated systems to control the speed and inclinations of the runway. It also comes equipped with shock grids at the start of the runway that can be turned on or off as required. The Motorized Running Wheel and the Positioning Running Wheel are apparatuses also used for exercise training. Other apparatuses that are used for the assessment of motor and locomotion functions include the Rotarod, the Grid Test, the Gait Test, and the Parallel Rod Test. (For more motor assessment apparatuses click here.)
Independent Lane Control: Each lane can counted separately.
Ultra Quiet: Reduce subject disruption
Speed: 0-80 meters per minute
Acceleration: Adjustable acceleration in 0.1 meter/minute increments
Slope: 0 to 25 degrees
Single Belt texture facilitates animal grip
Single-phase power system
A shock grid can be optionally inserted and can be controlled with our conductor software.
Shock intensity is adjustable and can be correlated to LED lamp to allow mice and rats to learn when the shock grid is active.
Design of shock grid carefully designed to avoid injuries to animals.
The grid can be removed for easy cleaning
163V, adjustable.
0 – 4mA adjustable in 0.1mA units
Tough Aluminum alloy frame
Clear acrylic walls
Black non-reflective separators
Display distance traveled
Display shock on/off
Manually start / stop switch of electrical stimulus system per lane
Manually start / stop of treadmill per lane
Manually adjust the slope
Air puff accessory for aversive stimuli also available upon request
A treadmill harness frame for motor testing and additional support is available upon request
MazeEngineers offers the Metabolic Treadmill variation for metabolic measurement in rodents.
Request a quote or inquire for details.
Convert your 5-lane mouse treadmill to a 3-lane rat treadmill for maximum versatility. Available on request.
The earliest use of treadmills can be traced back to the first century AD, when their typical use was for harnessing the power in systems like mills and manipulating heavy objects. The early design usually involved a wheel similar to something like the Rotarod or the Running Wheel which are often used in rodent-based motor function studies. Over the years the treadmill evolved to the modern-day horizontal runway. Initially, animals were used to drive treadmill-powered machinery. Eventually, with the growth of animal-based research, treadmills gained popularity as an exercise system.
Circular treadmills were often used for exercise treatments. These treadmills utilized a circular path whose belt speed could be varied as required. A study by Thompson and Stevenson (1965) based on this circular design investigated the temperature response of male Sprague-Dawley rats to different rates of exercise and the effects of anterior hypothalamic lesions. The investigation was able to observe the coping ability of the rats to the heat generated during the range of exercise.
Eventually, a treadmill design for small laboratory animals was described by Andrews (1965) that allowed the experimenter to change the speed of the runway between 13 to 70 ft/min and inclination between 0 to 16°. Unlike the previous circular design, Andrews’ treadmill made use of a horizontal plane that could be divided into individual lanes. The individual lanes provided the opportunity to train multiple animals at fixed training parameters such as speed and inclination.
The treadmill apparatus is constructed using aluminum and acrylic. It is available as a single-lane and as a five-lane system. The runway is made of a textured belt that facilitates animal grip. The individual lanes have a dimension of 44 x 6 x 12.7 cm and are separated by non-reflective black barriers. The speed of the lane can be varied between 0 to 80 meters per minute with acceleration increments of 0.1 meters/minute. The runway incline can be varied between 0 to 25 degrees. Each lane is equipped with a removable 12 x 6 cm shock grid that allows 0 to 4 mA electric shocks (adjustable in 0.1mA) and repetition rates of 1Hz, 2Hz, or 3Hz. An LED accompanies the shock grid to allow the subject to know when the shock grid is ON or OFF. Additionally, air puffs can also be added to the apparatus as an aversive stimulus. The treadmill comes with associated software to control different variables of the apparatus.
Vihko, Salminen, and Rantamäki’s 1978 study utilized the variable inclination ability of the horizontal treadmill to evaluate the oxidative and lysosomal capacity in mice. The study divided male NMRl mice into groups that were either trained at zero inclination or at an uphill inclination of 8 degrees at speeds of either 20 m/min or 25 m/min. The investigation revealed a slightly higher increase in oxidative capacity in subjects that ran uphill in comparison to zero inclination runners.
The use of treadmills in weight and diet-based studies is also a popular research area. Askew, Dohm, Weiser, Huston, and Doub (1980) investigated if supplemental dietary carnitine augmented fatty acid oxidation. The investigation involved exercising male Carworth CFN rats that were fed either normal, high fat, or identical diets supplemented with 1.0% D, L-carnitine on the treadmill. The study concluded that exercise or fat feeding-induced fatty acid oxidation demands were met by endogenous levels of carnitine and supplementation did not have any significant effect. Another diet and exercise based study was conducted by Bauer and Griminger (1983). Their study evaluated the effects of adequate, low and high calcium and phosphorous supplemented diet on bones and kidneys of female rats. It was observed that forced treadmill running could not compensate low or high intakes of either mineral though it did benefit long bone density.
Raab, Smith, Crenshaw, and Thomas (1990) investigated the bone mechanics of young and old Fischer 344 female rats using a shock grid equipped treadmill. The investigation was able to conclude the beneficial effects of the exercise was not limited by age. In comparison to the controls, both exercised young and old rats showed an exercise-induced increase in fat-free dry weight of the femur and to a certain extent in the humerus. MacNeil and Hoffman-Goetz’s (1993) study also showed that forced treadmill running resulted in the enhanced cytotoxic mechanism of natural immunity in male C3H mice, both in-vivo and in-vitro.
The physiological adaptations resulting from exercise were investigated by Moraska, Deak, Spencer, Roth, and Fleshner (2000). The research that involved training male Sprague-Dawley rats on a shock grid-equipped treadmill was able to observe both positive and negative physiological adaptations. While exercise resulted in decreased body weight gain and increased muscle citrate synthase activity, negative adaptations corresponding to chronic stress were also observed.
Exercise is also considered to have positive effects on cognitive abilities. Albeck, Sano, Prewitt, and Dalton (2006) were able to show that Brown Norway/Fisher 344 rats performed better in the Morris Water Maze task following mild forced treadmill running. Another similar study conducted by O’Callaghan, Griffin, and Kelly (2009) also showed the positive effects of long-term treadmill running on plasticity and growth factor expression affected by age.
Baek et al., (2012) investigated the effects of treadmill exercise rehabilitation on intracerebral hemorrhage (ICH). ICH-induced subjects that underwent exercise training starting on the fourth day after surgery showed a significantly higher immunofluorescent intensity of BDNF and p-Y705-TrkB. The study recommended early treadmill exercise as a potential promoter of the repair process in ICH.
The effects of treadmill training on mechanical allodynia were evaluated by Nees et al., (2016). The study involved female C57BL/6 mice that had undergone a moderate T11 contusion spinal cord injury. Subjects that underwent moderate treadmill training showed a significant reduction in response rate to light mechanical stimuli using Von Frey Filaments. Further, treadmill running also reduced CGRP-labeling density. Treadmill running has been shown as a potential therapeutic treatment to deal with chronic pain (Pitcher., 2018).
Clean the treadmill apparatus before and after use with each subject to prevent any lingering stimuli from influencing the subject performance. Ensure that the apparatus is appropriately lit, and the task is performed in an undisturbed environment to minimize the influence of any external stimuli on the performance. Tracking and recording of the performances can be done with the assistance of an external tracking and video system such as the Noldus EthoVision XT or ANY-Maze.
For exercise training, the subjects do not require any pretraining. However, subjects can be familiarized with the treadmill and the shock grids prior to the exercise regime to minimize novelty stress. Exercise training and duration are dependent on the type of investigation. Regardless of the training schedule, subjects must be given appropriate rest periods to prevent muscle fatigue.
Turn off the treadmill and place the subject on the runway belt of the treadmill. Immediately turn on the shock grid. Allow the subject to explore the lane unrestricted (at least 1 to 3 minutes) until it has explored the entire lane and/or received at least one shock. Following the exploration, turn on the treadmill, slowly increasing the speed until the runway begins moving. Ensure the subject is moving and not moving towards the shock grid; if not encourage the subject by tapping it with a wire brush or tail tickling. Continue to slowly increase the speed (up to 10 m/min) and stop the treadmill after 10 minutes have elapsed. Allow the subject to explore the lane before returning it to its cage. Consecutive sessions can be performed at higher increments of speeds for 15 minutes. Repeat the process as needed.
Place the subject on the treadmill and immediately turn on the shock grid. Start the treadmill at a predetermined speed and begin observation. Gradually increase the speed of the runway. Remove the subject if it remains within a one-body length area near the shock zone or on the shock grid (Fatigue zone) for more than 5 continuous seconds. Do not interrupt the test unless to remove the subject that has met the removal criterion.
Evaluation of the effects of forced treadmill running on neurotrophic factors in EAE
Patel and White (2013) evaluated the clinical effects of forced exercise in the experimental autoimmune encephalomyelitis (EAE) model of Multiple Sclerosis. Female Lewis rats were divided into 4 groups prior to EAE induction; EAE exercise, EAE sedentary, control exercise and control sedentary. Subjects in the forced exercise group underwent 5 days of treadmill habituation. Following habituation, subjects of the EAE group were intradermally injected with 50 µg of purified myelin oligodendrocyte glycoprotein (MOG33–55) dissolved in 50 µL saline homogenized (1:1 v/v) with Freund’s Complete (with mycobacteria) Adjuvant. After inoculation, exercise groups underwent 10 consecutive days of forced treadmill running. On the first 2 days, subjects were exercised in 60 minutes session consisting of two 30-minute trials at speeds of 15 m/sec and 30 m/sec, respectively. The following sessions were increased to 90 minutes. Data analysis revealed no significant differences in the disability scores within the EAE group. Further, brain tissue proteins analysis showed a higher concentration of tumor necrosis factor-α (TNF-α) of either EAE group in comparison to the controls. However, no significant difference in the concentration could be observed within the EAE group.
Investigation of low-intensity exercise effects on hippocampal parvalbumin immunoreactivity
Nguyen, Killcross, and Jenkins (2013) assessed the behavioral and morphological changes resulting from low-intensity treadmill exercise using male Long-Evan rats. Subjects were divided into two groups; the exercise group and the control group. The exercise group underwent 30 days of 30-minute running training at 5% inclination (5 m/min for the first 5 days and progressed to 10 m/min for the remaining days). Control group subjects were handled daily for 5 minutes during this period. Following the treatment, subjects were evaluated for sociability in the social interaction test, anxiety using the Elevated Plus Maze, and locomotion in the open field. A significant effect of exercise was observed in the exercised group that showed more passive interactions with the social stimulus. However, no effect of exercise could be observed on the locomotor activity and anxiety of the group. Brain analysis of the exercised group revealed a significant increase in the number of parvalbumin-immunoreactive neurons in the hippocampus localized to the CA1 and CA2/3 regions.
Investigation of therapeutic effects of moderate exercise in the PTSD model
Patki et al. (2014) evaluated the ameliorating effects of moderate treadmill exercise on post-traumatic stress-induced behavioral deficits. The investigation divided male Wistar rats into 4 groups; control group, exercised control, Single prolonged stress (SPS) group and SPS exercised group. The single prolonged stress treatment subjected the animals to one-time, consecutively applied stressed paradigm of 2-hour restraint, 20 minutes forced swim followed by after 15 minutes rest and finally ether anesthesia exposure until loss of consciousness. Following treatment, exercise group was exercised on the treadmill with the shock grids for 2 weeks following 10 m/min for 30 min/day (week 1), and 15 m/min for 30 min/day (week 2) regime. Subjects behavioral performances were evaluated using behavioral assays including the Radial Arm Maze, the Light-Dark Box, and the Sucrose consumption test. The exercise was found to have a beneficial impact of the animals subjected to SPS with relatively reduced anxiety-like behaviors and better memory when compared to SPS only treatment group.
Evaluation of the role of resistance and aerobic exercise training on insulin sensitivity measures
Hall et al. (2013) investigated the effects of different exercise training on glucose tolerance in Type 1 Diabetes Mellitus (T1DM) rats. Male Sprague-Dawley rats were divided into 5 experimental groups; control, diabetic control, diabetic resistance exercised, and diabetic high and low-intensity treadmill exercised. T1DM was induced in subjects by daily intraperitoneal streptozotocin injections (20 mg/kg) over 5 consecutive days. Subjects in the resistance exercise group were trained to climb a ladder with incremental loads while the treadmill groups were trained on 27 m/min (high intensity) or 15 m/min (low intensity) over 6 weeks. Data analysis showed significantly lower intravenous glucose tolerance test (AUC-IVGTT) values in all exercise group in comparison to the diabetic controls. Of the exercised groups, treadmill trained groups showed the lowest insulin dose requirement. Further, a significant reduction in insulin dosage was observed in the high-intensity treadmill trained group.
Evaluation of the effects of treadmill exercise on stress-coping strategies
Cigarroa et al., (2016) aimed to investigate the impact of obesity and its interaction with exercise on stress-coping behaviors. Female Sprague-Dawley rats were divided into the following eight groups based on diet and exercise combinations, sedentary-standard diet (SED-ST), sedentary-cafeteria diet (SED-CAF), control-standard diet (CON-ST), control-cafeteria diet (CON-CAF), treadmill-low intensity-standard diet (TML-ST), treadmill-low intensity-cafeteria diet (TML-CAF), treadmill-higher intensity-standard diet (TMH-ST), and treadmill-higher intensity-cafeteria diet (TMH-CAF). The low-intensity exercise group ran on the treadmill at 12 m/min while the high-intensity running group ran at 17 m//min for 8 weeks. Behavioral assessments of the groups showed that the cafeteria diet impaired stress-coping strategies as observed in the Shuttle-Box test. However, a partial recovery from the CAF diet-induced impairment was observed in the treadmill intervention groups.
Investigation of the effects of treadmill exercise on inflammatory and epigenetic parameters
Lovatel et al. (2013) evaluated the effects of aging and treadmill exercise on pro and anti-inflammatory cytokines levels, and activation of NF-kB and histone H4 acetylation levels in the hippocampus. Male Wistar rats aged 3 and 20 months old were divided into an exercise group and a sedentary group. Exercised animals were trained on the treadmill in 20 minutes sessions, every day for 2 weeks without any aversive motivation. The sedentary animals were also placed on the treadmill for 5 minutes without any running stimulus during the 2 weeks. The effects of exercise training were evaluated at 1 hour, 18 hours, 3 days and 7 days after the last session. Analysis of the Inhibitory Avoidance task performed on 14, 15, 17 or 21st days after exercise training revealed that the aged rats exhibited impaired performance in comparison to the young subjects. However, exercise training resulted in a significant increase in the step-down latency, without any delay effect after the last training session. It was observed that the exercised-aged animals exhibited lower TNF-α levels (compared to the sedentary group), had acute and delayed (18 hours) reduction in IL-1β levels and significantly decreased NF-kB levels. Further, exercise training resulted in a significant increase in IL-4 levels in young rats while H4 acetylation levels significantly increased in aged rats.
Investigation of protective effects of treadmill exercise in Alzheimer’s disease
Um, et al., (2011) evaluated the protective effects of exercise on neuronal cell death using Tg-NSE/hPS2m mice model of Alzheimer’s disease. Along with Tg-NSE/hPS2m mice, control non-Tg mice (all aged 24 months) were divided into sedentary non-Tg mice (non-Tg-SED), treadmill-exercised non-Tg mice (non-Tg-TE), sedentary Tg mice (Tg-SED) and treadmill exercised Tg mice (Tg-TE). The exercised group underwent 5 days of treadmill familiarization at 5 m/min for 10 min/day. The process was followed by 3 months of treadmill training at zero inclination, at a speed of 12 m/min for 60 minutes every day for 5 days. Evaluation of performance in the Water Maze task showed exercise-induced improvement in the cognitive functions of the rats. Further, brain analysis suggested a strong therapeutic potential of treadmill exercise to inhibit both Aβ-42 and neuronal death pathways.
Investigation of the effects of exercise on the progression of Parkinson’s disease
Toy et al., (2014) used male C57BL/6J and Drd2-eGFP-BAC mice to investigate the effects of treadmill running on the basal ganglia function and progression of Parkinson’s disease. Half of the subjects underwent 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) lesioning at a dose of 20 mg/kg injected intraperitoneally 4 times at intervals of 2 hours. Subjects were divided into four treatment groups; saline, saline + exercise, MPTP, and MPTP + exercise groups. Exercise training began 5 days after treatment (after cell death was complete) following a 5 days/week schedule for 6 weeks. Performance analysis showed that though initially, MPTP + exercise mice were slower than the saline + exercise mice, starting at week 5 no significant difference could be observed between the two groups. Further, it was observed that exercise led to increased dendritic spine density and arborization in striatal medium spiny neurons (MSNs) in the MPTP group. Exercise also led to increased expression of synaptic proteins PSD-95 and synaptophysin.
Evaluation of the effects of forced treadmill running in stroke recovery
Cechetti et al., (2012) investigated the therapeutic effects of treadmill running in male Wistar rats subjected to chronic cerebral hypoperfusion. Subjects were divided into a pre-surgery exercise group, a post-surgery exercise group, and a pre + post-surgery exercise group. Corresponding sedentary groups were used as controls. Subjects were exercised on the treadmill at gradually increasing speeds and time for 12 weeks (3 sessions per week). Analysis of memory and learning in the Morris Water Maze suggested a reversal of cognitive deficits in the post and pre + post ischemic group that received forced treadmill training. Further, exercise also led to the regulation of oxidative damage and antioxidant enzyme activity in the hippocampus suggesting a neuroprotective effect.
Investigation of therapeutic effects of moderate exercise on oxidative stress-mediated anxiety
Male Sprague-Dawley rats were divided into sedentary control, exercise control, sedentary with 7 days l-Buthionine-(S,R)-sulfoximine (BSO; 300 mg/kg body weight i.p) treatment, and exercise plus 7 days BSO (300 mg/kg body weight i.p) treatment. Exercise training was carried out over 4 weeks at zero inclinations at 15 m/min for 30 minutes in the first week and 60 minutes in the remaining weeks. Anxiety behaviors were observed in the Open-Field and the Light-Dark box tasks. Data analysis suggested that treadmill exercise prior to BSO treatment prevented anxiety-like behaviors and an increase in oxidative stress in the exercised group. (Salim et al., 2010)
Investigation of exercise effects on short-term memory deteriorated by maternal LPS exposure
Pregnant Sprague-Dawley rats were divided into two groups, a control group, and the lipopolysaccharide-exposed (LPS) group. The LPS group received 1 mL intracervical injects of 0.15 mg/kg LPS suspended in pyrogen-free saline (PFS), while controls received PFS on days 15, 17 and 20 of pregnancy. Pups obtained from these pregnancies were then divided based on the exercise treatment into the control group, the mild-intensity exercise group, the moderate-intensity exercise group, the maternal LPS-exposed group, the maternal LPS-exposed + mild-intensity exercise group, and the maternal LPS-exposed + moderate-intensity exercise group. Exercise training involved running on a zero inclination treadmill at the speed of 8 m/min (mild-intensity) or 14 m/min (moderate-intensity) for 30 minutes, 5 times a week for 4 weeks. Maternal LPS-exposed rats showed a decrease in step-down latency in step-down avoidance. However, mild-intensity and moderate-intensity treadmill exercises led to improved step-down latencies in these rats (Kim et al., 2015).
The treadmill apparatus is used for forced exercise training in rodents. It can also be used to compare performance differences between subjects of different strains, ages, and sexes. Effects of diseases and disorders on the physical activity capacity and the subject’s motor and locomotion capabilities can also be evaluated in the treadmill apparatuses. Further, the effects of pharmacological manipulations, lesions and other investigatory treatments such as stress, on the treadmill performances can also be assessed.
The following observations can be made using the treadmill apparatus,
Running time
Distance traveled
Maximum speed reached
Number of aversive stimulations
Average position on the runway
Time to fatigue/exhaustion
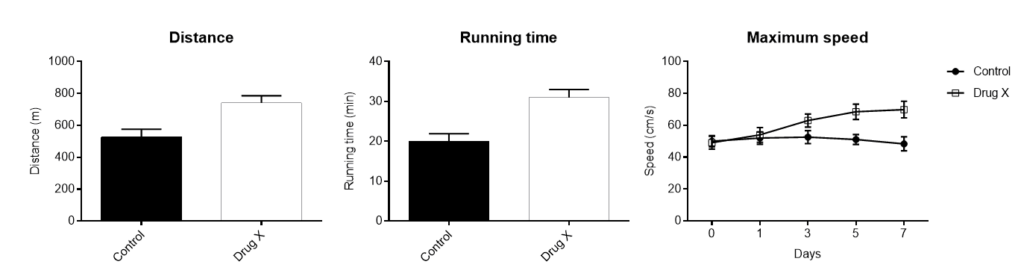
Marcaletti et al., (2011) recommend a group size of 10 to 12 animals to enable sufficient statistical power.
Goutianos et al., (2015) compared the treadmill performances of human and rat subjects. The study was conducted to understand the extent to which the rodent models of exercise can be translated to human applications. Male Wistar rats were trained at a starting speed of 20 m/min which was gradually increased to a speed corresponding to 86% of maximal running velocity within approximately 30 seconds. A similar exercise protocol was applied to male, nonsmokers, and recreationally active human subjects till they reached 86% of maximal running velocity. No significant difference in time to exhaustion was observed between either subject group. Further, analysis of pre and post-exercise blood samples of the subjects showed similar qualitative (26/30 biochemical blood variables) and quantitative (7.9% in rats and 7.3% in humans) characteristics.
Another area of interest is the use of physical exercise to improve the ability to cope with diseases and their potential benefits for recovery in humans. The study conducted by Miki Stein, Munive, Fernandez, Nuñez, and Torres Aleman (2017) aimed to evaluate the potential of exercise as a protective measure against Alzheimer’s disease. Observation of treadmill performances of male adult C57BL/6J mice and Alzheimer’s animal model APP/PS1 mice showed exercise as a potential diagnostic tool.
Lawson et al., (2014) utilized the light-induced retinal degeneration (LIRD) model to investigate the potential protective effects of aerobic exercise on retinal neurons. Their experiment trained BALB/cJ and BALB/cAnNCrl mice on the treadmill 2 weeks prior to exposing them to either 10,000 lux (bright condition) or 25 lux (dim condition) light for 4 hours. Following light exposure, subjects were additionally trained on the treadmill for another 2 weeks. Analysis of retinal function and photoreceptor numbers revealed that exercised mice had greater values for both parameters as opposed to sedentary control, both exposed to bright light. Further, it was also observed that BDNF signaling mediated the neuroprotective effects of treadmill running.
Strengths
The treadmill is a straightforward and simple apparatus that can be used for forced exercise training of rodents. The multiple runway treadmill allows observation and comparison of performances of a group of animals at a time. The treadmill can be used in the assessment of the physical activity of the subjects and the effects of pharmacological manipulations on the same. Further, the effects of neurological diseases, neuropsychiatric diseases, and lesions on physical activity and locomotion capabilities can also be evaluated. The most common use of the treadmill is, however, to investigate the therapeutic effects of exercises. The treadmill apparatus allows exercise training at variable angles and speeds. Subjects can be forced trained with the help of the available shock grids in the apparatus or using other kinds of motivations such as air puffs. The apparatus can also be easily adapted for different investigations.
Limitations
The treadmill relies on the locomotion and motor capabilities of the subjects. Therefore the subjects with serious deficits in these abilities are not suitable for this task. The subject’s strain, age, and gender can also affect training performance. The weight of the subject is also a crucial parameter that must be considered while deriving data from the treadmill task. Though habituation to the treadmill isn’t necessary, it is recommended to avoid stress resulting from novelty. The animal’s emotional state and explorative drive may also impact running performances. The use of electric shocks as an aversive stimulus in the treadmill may potentially stress the animals and affect the results of the task. The treadmill may not be as effective as other exercise systems such as the Positioning Running Wheel when it comes to exercise training (Chen, Yang & Chang, 2016). Using the treadmill to assess training endurance may not be beneficial with repeated trials (Knab et al., 2009). Overtraining the subjects on the treadmill can result in muscle fatigue. Thus appropriate rest periods should be included within the training regime.
Albeck, D. S., Sano, K., Prewitt, G. E., & Dalton, L. (2006). Mild forced treadmill exercise enhances spatial learning in the aged rat. Behavioural Brain Research, 168(2), 345–348. doi:10.1016/j.bbr.2005.11.008
Andrews, R. J. (1965). Treadmillfor small laboratory animals. Journal of Applied Physiology,20(3):572-4.
Askew, E. W., Dohm, G. L., Weiser, P. C., Huston, R. L., & Doub, Jr., W. H. (1980). Supplemental Dietary Carnitine and Lipid Metabolism in Exercising Rats. Annals of Nutrition and Metabolism, 24(1), 32–42. doi:10.1159/000176314
Baek, S.-S., Jun, T.-W., Kim, K.-J., Shin, M.-S., Kang, S.-Y., & Kim, C.-J. (2012). Effects of postnatal treadmill exercise on apoptotic neuronal cell death and cell proliferation of maternal-separated rat pups. Brain and Development, 34(1), 45–56. doi:10.1016/j.braindev.2011.01.011
Bauer, K. D., & Griminger, P. (1983). Long-term effects of activity and of calcium and phosphorus intake on bones and kidneys of female rats. Journal of Nutrition, 113(10):2011-21.
Brossia-Root, L. J., Alworth, L. C., & Malek, M. H. (2016). Considerationsfor aerobic exercise paradigms with rodent models. Lab Animal, 45(6):213-5. doi: 10.1038/laban.1023.
Cechetti, F., Worm, P. V., Elsner, V. R., Bertoldi, K., Sanches, E., … Netto, C. A. (2012). Forced treadmill exercise prevents oxidative stress and memory deficits following chronic cerebral hypoperfusion in the rat. Neurobiology of Learning and Memory, 97(1), 90–96. doi: 10.1016/j.nlm.2011.09.008
Chen, C.C., Yang, C.L., & Chang, C.P. (2016). An Innovative Running Wheel-based Mechanism for Improved Rat Training Performance. Journal of Visual Experiments, (115). doi: 10.3791/54354.
Cigarroa, I., Lalanza, J. F., Caimari, A., del Bas, J. M., Capdevila, L., Arola, L., & Escorihuela, R. M. (2016). Treadmill Intervention Attenuates the Cafeteria Diet-Induced Impairment of Stress-Coping Strategies in Young Adult Female Rats. PLOS ONE, 11(4), e0153687. doi:10.1371/journal.pone.0153687
Costa, M. S., Ardais, A. P., Fioreze, G. T., Mioranzza, S., Botton, P. H., …, Porciúncula, L. O. (2012). Treadmill running frequency on anxiety and hippocampal adenosine receptors density in adult and middle-aged rats. Progress in Neuro-psychopharmacology & Biological Psychiatry, 36(1):198-204. doi: 10.1016/j.pnpbp.2011.10.015.
Dougherty, J. P., Springer, D. A., & Gershengorn. M. C. (2016). The treadmill Fatigue Test: A Simple, High-throughput Assay of Fatigue-like Behavior for the Mouse. Journal of Visual Experiments, (111). doi: 10.3791/54052.
English, A. W, Wilhelm, J. C., & Sabatier, M. J. (2011). Enhancing recovery from peripheral nerve injuryusing treadmill training. Annals of Anatomy, 193(4):354-61. doi: 10.1016/j.aanat.2011.02.013.
Goutianos, G., Tzioura, A., Kyparos, A., Paschalis, V., Margaritelis, N. V., … Vrabas, I. S. (2015). The rat adequately reflects human responses to exercise in blood biochemical profile: a comparative study. Physiological Reports, 3(2), e12293. doi:10.14814/phy2.12293
Hall, K. E., McDonald, M. W., Grisé, K. N., Campos, O. A., Noble, E. G., & Melling, C. W. J. (2013). The role of resistance and aerobic exercise training on insulin sensitivity measures in STZ-induced Type 1 diabetic rodents. Metabolism, 62(10), 1485–1494. doi:10.1016/j.metabol.2013.05.012
Hwang, D. S., Kwak, H. B., Ko, I. G., Kim, S. E., Jin, J. J., …, Kwon, O. Y. (2016). Treadmill Exercise Improves Memory Function Depending on Circadian Rhythm Changes in Mice. International Neurourology Journal, 20(Suppl 2):S141-149.
Kim, K., Sung, Y. H., Seo, J. H., Lee, S. W., Lim, B. V., Lee, C. Y., & Chung, Y. R. (2015). Effects of treadmill exercise-intensity on short-term memory in the rats born of the lipopolysaccharide-exposed maternal rats. Journal of Exercise Rehabilitation, 11(6): 296-302. DOI: https://doi.org/10.12965/jer.150264
Knab, A. M., Bowen, R. S., Moore-Harrison, T., Hamilton, A. T., Turner, M. J., & Lightfoot, J. T. (2009). Repeatability of exercise behaviors in mice. Physiology & Behavior, 98(4):433-40. doi: 10.1016/j.physbeh.2009.07.006.
Lawson, E. C., Han, M. K., Sellers, J. T., Chrenek, M. A., Hanif, A., …, Pardue, M. T. (2014). Aerobic exercise protects retinal function and structure from light-induced retinal degeneration. Journal of Neuroscience, 34(7):2406-12. doi: 10.1523/JNEUROSCI.2062-13.2014.
Lovatel, G. A., Elsner, V. R., Bertoldi, K., Vanzella, C., Moysés, F. dos S., … Siqueira, I. R. (2013). Treadmill exercise induces age-related changes in aversive memory, neuroinflammatory and epigenetic processes in the rat hippocampus. Neurobiology of Learning and Memory, 101, 94–102. doi: 10.1016/j.nlm.2013.01.007
MacNeil, B., & Hoffman-Goetz, L. (1993). Chronic exercise enhances in vivo and in vitro cytotoxic mechanisms of natural immunity in mice. Journal of Applied Physiology, 74(1), 388–395. doi:10.1152/jappl.1993.74.1.388
Miki Stein, A., Munive, V., Fernandez, A. M., Nuñez, A., & Torres Aleman, I. (2017). Acute exercise does not modify brain activity and memory performance in APP/PS1 mice. PLoS One, 2017 May 22;12(5):e0178247. doi: 10.1371/journal.pone.0178247.
Moraska, A., Deak, T., Spencer, R. L., Roth, D., & Fleshner, M. (2000). Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 279(4), R1321–R1329. doi:10.1152/ajpregu.2000.279.4.r1321
Nees, T. A., Tappe-Theodor, A., Sliwinski, C., Motsch, M., Rupp, R., …, Blesch, A. (2016). Early-onset treadmill training reduces mechanical allodynia and modulates calcitonin gene-related peptide fiber density in lamina III/IV in a mouse model of spinal cord contusion injury. 157(3):687-97. doi: 10.1097/j.pain.0000000000000422.
Nguyen, J. C. D., Killcross, A. S., & Jenkins, T. A. (2013). Effect of low-intensity treadmill exercise on behavioural measures and hippocampal parvalbumin immunoreactivity in the rat. Behavioural Brain Research, 256, 598–601. doi: 10.1016/j.bbr.2013.09.004
O’Callaghan, R. M., Griffin, É. W., & Kelly, Á. M. (2009). Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus, 19(10), 1019–1029. doi:10.1002/hipo.20591
Patel, D. I., & White, L. J. (2013). Effect of 10-day forced treadmill training on neurotrophic factors in experimental autoimmune encephalomyelitis. Applied Physiology, Nutrition, and Metabolism, 38(2), 194–199. doi:10.1139/apnm-2012-0303
Patki, G., Li, L., Allam, F., Solanki, N., Dao, A. T., …, Salim, S. (2014). Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiology & Behavior, 130, 47–53. doi:10.1016/j.physbeh.2014.03.016
Pitcher, M. H. (2018). The Impact of Exercise in Rodent Models of Chronic Pain. Curr Osteoporos, doi: 10.1007/s11914-018-0461-9.
Raab, D. M., Smith, E. L., Crenshaw, T. D., & Thomas, D. P. (1990). Bone mechanical properties after exercise training in young and old rats. Journal of Applied Physiology, 68(1), 130–134. doi:10.1152/jappl.1990.68.1.130
Salim, S., Sarraj, N., Taneja, M., Saha, K., Tejada-Simon, M. V., & Chugh, G. (2010). Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behavioural Brain Research, 208(2), 545–552. doi: 10.1016/j.bbr.2009.12.039
Thompson, G. E., & Stevenson, J. A. F. (1965). THE TEMPERATURE RESPONSE OF THE MALE RAT TO treadmill EXERCISE, AND THE EFFECT OF ANTERIOR HYPOTHALAMIC LESIONS. Canadian Journal of Physiology and Pharmacology, 43(2), 279–287. doi:10.1139/y65-027
Toy, W. A., Petzinger, G. M., Leyshon, B. J., Akopian, G. K., Walsh, J. P., … Jakowec, M. W. (2014). Treadmill exercise reverses dendritic spine loss in direct and indirect striatal medium spiny neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurobiology of Disease, 63, 201–209. doi: 10.1016/j.nbd.2013.11.017
Um, H.-S., Kang, E.-B., Koo, J.-H., Kim, H.-T., Jin-Lee, … Cho, J.-Y. (2011). Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease. Neuroscience Research, 69(2), 161–173. doi: 10.1016/j.neures.2010.10.004
Vihko, V., Salminen, A., & Rantamäki, J. (1978). Oxidative and lysosomal capacity in skeletal muscle of mice after endurance training of different intensities. Acta Physiologica Scandinavica, 104(1), 74–81. doi:10.1111/j.1748-1716.1978.tb06253.x
| Weight | 50 lbs |
|---|---|
| Dimensions | 80 × 75 × 45 cm |
| Material | Aluminum alloy, Clear acrylic, Black non-reflective separators |
| Color | Black |
| Power/Voltage | 163V |
| Species | Mouse, Rat |
| speed_range | 0-80 meters per minute |
| acceleration_increments | 0.1 meter/minute increments |
| slope_range | 0 to 25 degrees |
| shock_current_range | 0 – 4mA adjustable in 0.1mA units |
| power_system | Single-phase power system |
| lane_options | ['1 lane', '5 lane'] |
| independent_lane_control | Each lane can be counted separately |
| motor_type | Ultra Quiet |
| belt_texture | Single Belt texture facilitates animal grip |
| shock_grid | Optional, removable for cleaning |
| shock_led_correlation | Adjustable shock intensity correlated to LED lamp |
| conversion_option | 5-lane mouse to 3-lane rat conversion available |
| Device Type | 1 lane, 5 lane |
| Size | Mouse, Rat |