$810.00
Ophthalmic surgery, also known as ocular surgery, is performed on the eye specifically upon the external surface of the cornea.

RWD is a global leader in the design and manufacturing of advanced research and laboratory equipment. Specializing in high-precision instruments for neuroscience, behavioral science, and pharmacology.

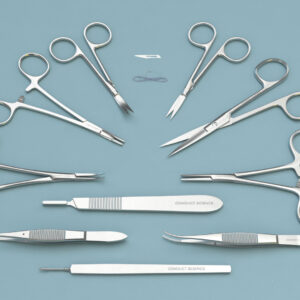
Model | Description | Quantity |
|---|---|---|
RWD-S11001-08 | VANNAS Spring Scissors (Triangular) -S/S Str/5*0.1mm/8.5cm | 1 |
RWD-S11002-08 | VANNAS Spring Scissors (Triangular)-S/S Cvd/5*0.1mm/8.5cmm | 1 |
RWD-S11003-08 | VANNAS Spring Scissors (Triangular)-S/S ANGLED/6.3*1.35MM/8.5CM | 1 |
RWD-S12005-10 | IRIS-Fine Scissors (Round Type) S/S Str/23*5mm/10.5cm | 1 |
RWD-S12006-10 | IRIS-Fine Scissors (Round Type) S/S Cvd/22*4.5mm/10.5cm | 1 |
RWD-S15006-09 | O’BRIEN Stitch Scissors S/S Angled/13.5*4mm/9.5cm | 1 |
RWD-F12013-10 | Dressing Forceps w/o Serrations 45°Cvd, S/S, 10cm | 1 |
RWD-F13019-12 | ADSON 1×2 Teeth Tissue Forceps Str, 0.8mm Tips, 10cm | 1 |
RWD-F12005-10 | IRIS Dissecting Forceps Str, 0.8mm Tips, 10cm | 1 |
RWD-F12006-10 | IRIS Dissecting Forceps light Cvd 0.8mm Tips, 10cm | 1 |
RWD-F18011-09 | McPHERSON Forceps w/5mm Platform-Str, Tip0.4*0.9mm, 9cm | 1 |
RWD-F31047-12 | OLSEN-HEGAR Needle Holders with Scissors-Str, 10*2.15mm/12cm | 1 |
RWD-F35401-50 | NA Terylene Suturesw/Needle-o3/8/3x10/90cm/5-0 (50/Box) | 0.2 |
RWD-R21027-12 | STEVENS Hooks, 1Angle Tooth, 5mm depth, 12.5cm | 1 |
RWD-R22029-04 | Colibri Eye Specula-15mm spread, 4cm | 1 |
RWD-SP0001-P | Instrument Storage portfolio | 1 |
Ophthalmic surgery, also known as ocular surgery, is performed on the eye specifically upon the external surface of the cornea. A thorough and detailed ophthalmic examination provides a rapid and accurate diagnosis of many ophthalmic diseases. Research in vision and ophthalmology improves treatment and quality of life in humans as well as in animals. This improvement stems from advancement in diagnosis and treatment of human and animal diseases and disability. As vision research is aiming at revealing and understanding the morphology and physiology of complex and interconnected biological systems, research on living animals is essential to continue progress in many areas of clinical and basic ophthalmic research.
Earlier animal models of ophthalmic surgery included dogs and cats due to their large eye sizes. However, these models have their shortcomings which include the husbandry cost, handling difficulties and lack of availability of transgenic animals. These disadvantages led to the popularity of rodents as the new model organisms in ophthalmic research. Among rats and mice, the latter has seen more popularity due to the availability of a range of genetically modified mice and the existence of an extensive array of reagents that can be used to further define responses in this species. Rodents, as opposed to the previously used animal models, have shorter breeding cycles and faster regeneration. These qualities combined with their lower costs and smooth handling make them ideal for ophthalmic research.
Before any surgical procedure, it is necessary to ensure that the apparatus and the equipment used are thoroughly cleaned and sterilized. The instruments can be sterilized by using heat (e.g., autoclave or bead sterilizer), gas (ethylene oxide), or chemical methods (limited to 2% glutaraldehyde). Also, the operation area should be cleaned and sterilized properly.
Record the subject’s identification details such as strain and gender, and most importantly note the weight of the subject. Also, make a physical assessment of the subject for its health status and activeness. Ensure that the subject has been appropriately acclimated to the facility. The acclimation process can last from a few days to a couple of weeks.
Anesthesia is induced in the subject via inhalant agents with the help of anesthetic systems that use a face mask or an anesthetic chamber. The dose of the anesthetic agent and the duration of induction are dependent on the weight of the subject among other factors. Once the anesthesia is induced, the depth of anesthesia can be assessed using the toe pinch test. Additionally, physiological parameters can be monitored throughout the procedures to ensure that the anesthesia is effective.
In Vivo Ocular Enucleation Protocol (Aerts et al., 2014)
Ex-orbital and Intra-orbital Lacrimal Gland Excision Protocol (Shinomiya et al., 2018)
Murine Corneal Transplantation Protocol (Yin et al., 2014)
Corneal grafting
Obtaining the donor corneal button
Graft bed preparation
Graft suturing
Deepening the anterior chamber
Suture removal
Composite Orbital and Periorbital Allotransplantation Protocol (Zor & Karagoz, 2015)
The variable bypass vaporizers are the most commonly used vaporizes. Their working principle involves splitting the fresh gas flow and saturating a small portion completely with the volatile anesthetic before recombining into the main gas flow. This process is achieved by setting of the anesthetic concentration using the control dial and the pressurized chamber of the plenum vaporizers. These devices are also equipped with thermo-compensation capabilities for a steady vaporizer output.
Preventive measures for postoperative pain in the subject can be done by the administration of opioids such as buprenorphine before incision. After the surgery, the subject must be kept warm in a recovery unit using hot water blankets, hot water bottles, heat pads, and warm sterile saline before returning to its home cage. Recovery from anesthesia should be monitored closely and respiratory support, if needed, should be provided. Analgesic should be maintained postoperatively for up to 48 hours in increments of 24 hours or as required. The subject can be returned to its home cage once it has recovered.
Check the wound redness, swelling or purulent discharge for at least 5 days. Also, monitor the body weights of the subjects daily. Rarely, postoperative prophylactic antibiotics can be administered to prevent infections. Euthanize the subject in case of an infection.
Study of Retinal Ganglion Cell Survival in an Optic Nerve Crush Injury Murine Model
Zhongshu Tang et al. conducted a detailed study on retinal ganglion cell survival using an optic nerve crush injury murine model. In the experiment, axonal degeneration followed by the progressive death of retinal ganglion cells (RGCs) resulted after optic injury, consequently leading to an irreversible vision loss. Traumatic optic neuropathy and optic nerve degeneration in glaucoma are examples of such diseases in humans. It is distinguished by regular transformations in the optic nerve head, progressive optic nerve degeneration, and loss of retinal ganglion cells.
The ophthalmic surgery kit is utilized to create the optic nerve crush (ONC) injury mouse model, which is an essential experimental disease model for traumatic optic neuropathy, and glaucoma. In this model, the crush injury to the optic nerve leads to progressive apoptosis of retinal ganglion cells. This disease model is widely used to investigate the general processes and mechanisms of neuronal death and survival, which is vital to the development of therapeutic measures.
Performing In Vivo Ocular Enucleation in the Mouse after Eye Opening
In the experiment, a more comfortable and straightforward technique for the removal of one or both eyes is designed and validated for mice older than 20 days. Summarily expressed, curved forceps were used to clamp the optic nerve behind the eye. Then circular movements were performed to limit the optic nerve, and the eyeball was removed. The strength of this technique is high reproducibility, minimal to no bleeding, rapid postoperative recovery and a very low learning threshold for the researcher.
Eye enucleation in rodents and many species are widely performed using different methods, which often involve the removal of the eyelids and cutting the optic nerve. These methods are more invasive and have a higher learning curve than the technique used in the experiment. Without removing or suturing the eyelids, the post-surgery recovery time is depreciated, resulting in higher animal welfare and more reproducible results. The in vivo enucleation technique employed in the research has been successfully applied with minor modifications in rats and appears useful to study the afferent visual pathway of rodents in general.
Development of a Dry Eye Mouse Model Produced by Ex-orbital and Intra-orbital Lacrimal Gland Excision
Katsuhiko Shinomiya et al. created a new dry eye model by exorbital and intraorbital lacrimal gland excision. The chronic dry eye is an increasingly prevalent condition worldwide, causing loss of vision and adversely affecting the quality of life. They have developed an improved surgical mouse model for the dry eye based on severe aqueous fluid deficiency, by excising both the exorbital and intraorbital lacrimal glands (ELG and ILG, respectively) of mice. After ELG plus ILG excision, dry eye symptoms were assessed using fluorescein infiltration observation, tear production measurement, and histological analysis of ocular surface. Tear production in the model mice was significantly diminished compared with the controls. Histological examination revealed significant severe inflammatory changes in the cornea, conjunctiva or meibomian glands of the model mice after surgery. The rodent ophthalmic model is useful for investigating both pathophysiologies as well as new therapies for the tear-volume-reduction type dry eye.
Evaluation of Ocular Ischemic Syndrome
The ocular ischemic model was designed to characterize the functional and morphologic changes caused by Bilateral Internal Carotid Artery Occlusion (BICAO). In the experiment, adult mice underwent BICAO or sham surgery. Variations in ocular blood flow and retinal circulation after surgery were investigated by MRA to verify the retinal blood flow occlusion. The images were taken to measure the thicknesses of the various retinal layers, and then the nucleus from the eyes was removed, and the enucleated eyes were embedded in paraffin for morphological and histological studies. The MRA images suggested that the ligation of both internal carotid arteries significantly diminished ocular blood flow and narrowed the blood vessels. Sham surgery or BICAO for seven days was evaluated by both ocular fundus photography and fluorescein angiography finding. The total retinal thickness and retinal ganglion cell density were reduced as compared with the sham group. However, no such variations were evident in the IPL layer in BICAO group, which was according to the results obtained from OCT images. The researchers successfully visualized the occlusion of blood flow after the BICAO by MRA, and angiography could also demonstrate that BICAO for 7 d was sufficient to maintain the retinal ischemia and induce the morphological changes. The protocol might be used as a mouse model for OIS in the future (Ling et al., 2017).
Designing Composite Orbital and Periorbital Allotransplantation Model
Vascularized composite allotransplantation has opened new avenues in reconstructive surgery and provided the researchers to reconstruct the defective tissue with the same tissue. The anatomic and histologic features of the eyeball are unique, and it is impossible to reconstruct the globe. The advancement in allotransplantation provided an option for the transplantation of eyeball. In the experiment, an orbital composite tissue allotransplantation model is designed. The results were encouraging and suggested that periorbital tissues together with the globe could be transplanted to another animal, even if they are not functional. However, a functional repair of the optic nerve might be possible in the future, given the significant advances in the field of neuro-ophthalmic, namely on the survival of the retinal ganglion cells and regeneration of the optic nerve. The allotransplantation model designed is providing essential hints about the issues that need to be studied before eye transplantation. Periorbital tissues with the globe can be transplanted from one individual to another, and the flap can survive, but the regeneration of the optic nerve regeneration and the survival of retinal ganglion cells are still the challenges that need to be overcome.
Evaluating Solid Organ Transplantation in Murine Corneal Transplantation Model
Several animal models exist for corneal transplantation and mice are the commonly used species. The strengths of using mice are the relative cost, the existence of various strains (genetically defined) that enable the researchers to study the immune responses, and the occurrence of an extensive array of reagents that can be used to define responses in this species further. The model created by ophthalmic surgery has defined factors in the cornea that are responsible for the relative immune privilege status of the ophthalmic tissues enabling corneal allografts to survive acute rejection in the absence of immunosuppressive therapy. The murine corneal transplantation model has also been used to define those factors that are most important in rejection of such allografts. Consequently, concerning mechanisms of both corneal allograft acceptance and rejection are understood studying murine models of corneal transplantation. The model allows the understudies to test various therapeutic strategies concerning eye diseases in an animal which have an immune system similar to that of humans. To that end, the existence of many reagents that react with murine factors as well as transgenic and gene-targeted mice permits evaluation of many more factors that would be the case with other species. This ability to evaluate numerous different factors, essential to both the success and failure of corneal allografts, is an added benefit of using the murine model as compared to other animal models in which surgery is more comfortable to perform due to the increased size of the eyes of the species.
Antifibrotic Drug Evaluation in Glaucoma Filtration Surgical Mouse Model
Glaucoma is an optic neuropathy, which leads to blindness if left untreated. The most common risk factor in glaucoma is elevated intraocular pressure (IOP), which can be counteracted surgically by filtration surgery. The postoperative subconjunctival scarring response, however, remains the primary obstacle to achieving long-term surgical success. Anti-tumor agents such as mitomycin C is widely used to avoid postoperative subconjunctival scarring. The model of glaucoma filtration surgery in the mouse has shown that the mouse model typically scarred within 14 d, but when augmented with mitomycin C, animals maintained lower IOP for a more extended period. The blebs following mitomycin C treatment resembled morphologically well-documented clinical observations confirming the validity and clinical relevance of this model. The anti-scarring action of mitomycin C is likely to be due to its effects on conjunctival fibroblast proliferation, apoptosis and collagen deposition and the suppression of inflammation. The data supported the suitability of the glaucoma filtration surgery mouse model for studying the wound healing response in glaucoma filtration surgery, and as a potentially useful tool for the in vivo evaluation of anti-fibrotic therapeutics in the eye (Seet et al., 2011).
The eye is a delicate organ, and requires extreme care before, during, and after a surgical procedure. An expert surgeon should select the appropriate surgical procedure and perform effective eye care. A complete understanding of eye functioning and ocular diseases is necessary to perform ophthalmological operations. The researcher needs to address three critical issues: First, ocular pain and blindness may endanger animal welfare. Second, several ocular diseases are vital signs of systemic disease with significant implications both for pets and laboratory animals. Third, eye diseases may complicate research efforts.
An appropriate strain and breed of the laboratory animal must be selected depending on the requirements of the investigation since the subject’s strain affects the ophthalmic manipulations. Other factors to be considered when selecting the subject are age and gender. Both these aspects influence eye physiology and morphology. Prepare a separate area from the surgical area for pre-operative preparations such as fur removal or hair trimming from the surgical site. The separate preoperative area is vital to eliminate contamination of the operative area. Before beginning the surgical procedure, ensure that all the equipment and the instruments are thoroughly cleaned and sterilized. Make sure that the subject is adequately anesthetized before beginning surgery. During surgery take care not to damage the surrounding tissues and muscles. Prepare an appropriate post-operative recovery area for the subject. Home cages that are to be used after the subject has recovered from surgery should be clean to avoid infecting the surgery area.
Topical application of the drugs may cause systemic absorption more relative to the size of the animal. Systemic absorption of the drugs may interfere with both the treatment of ocular disease and potential side effects, as the drug could act through circulating blood levels as well as by direct ocular penetration. The orbital vascular plexus, present in rodents and lagomorphs, differs substantially between species and understanding its anatomy is essential in orbital surgery and enucleation. Also, when evaluating ocular findings in experimental species derived from laboratory strains, the prevalence of inherited disease must be seen as a background against which other ocular diseases are noted. Background disease prevalence is particularly crucial among inbred strains, in which recessive genes may occur in a given strain not being used to study that specific trait.
Also, look out for the opening of sutures and displacement of pins after the surgery. Follow appropriate pain management protocols to avoid unnecessary discomfort for the subject. Care must be taken when translating results from rodent models of ophthalmic surgery to humans given their small size and skeletal differences
Rodent models for ophthalmic surgery offer many advantages as compared to larger animals like cats and dogs. Despite the advantage of larger size, the handling and maintenance of large animals outweigh their strengths for being used as models in research. Additionally, the cost of husbandry is high in comparison to rodents. Also, the transgenic animals are not readily available for the larger animals. Rodents, on the other hand, are economical since they are inexpensive and have shorter breeding cycles. Further, rodents are well-researched animals, and their biological processes and responses to diet modifications and drug administration are well-documented. The availability of athymic, transgenic, and knock-out rodents also makes them a viable choice for biomedical investigations.
However, there are significant anatomical, physiological and pathobiological differences between the eyes of the dog and cat and those of the rabbit, guinea pig, mouse and rat which have substantial implications for the investigation of ophthalmic conditions in these animals. For pathological studies, the precorneal tear film glands in rodents may prolapse. Similarly, the orbital vascular plexus, present in rodents and lagomorphs, differs substantially between species and the knowledge of its anatomy is essential in orbital surgery and enucleation. Also, the small size of the globe in many species may complicate the procedures. Investigating conjunctivitis in rodents requires a full and thorough history and clinical examination of individual animals as well as of the group since the conjunctivitis is common in rodents and may be influenced by the environmental conditions, which may interfere with the undergoing ophthalmic research. Many laboratory rodents possess red crusting around their eyes in cases of ocular irritation, upper respiratory tract infection, and stress. Porphyrin pigmented tears in normal amounts are produced by the Harderian glands in several rodent species but mainly by the rat, also some other rodent species. Diseases such as mycoplasmosis and sialodacryoadentitis (SDA), nutritional deficiencies, and other physiologic stresses are the factors that may cause chromodacryorrhea in rodent models for ophthalmic research (Williams, 2007). Appropriate remedial action to remove the stressors (infections, environmental or management conditions) should be taken
Aerts, J., Nys, J., & Arckens, L. (2014). A Highly Reproducible and Straightforward Method to Perform In Vivo Ocular Enucleation in the Mouse after Eye Opening. J Vis Exp, 92.
Ling, Y., Fu, Z., & Wang, Y. (2017). Surgical model for ocular ischemic syndrome in mice. Biomedical Research, 28(14).
Seet, L.-F., Lee, W. S., Su, R., Finger, S. N., Crowston, J. G., & Wong, T. T. (2011) Validation of the Glaucoma Filtration Surgical Mouse Model for Antifibrotic Drug Evaluation. Mol Med, 17(5-6), 557-567.
Shinomiya, K., Ueta, M., & Kinoshita, S. (2018). A new dry eye mouse model produced by exorbital and intraorbital lacrimal gland excision. Scientific reports, 8.
Tang, Z., Zhang, S., Lee, C., Kumar, A., Arjunan, P., Li, Y., . . . Li, X. (2011). An Optic Nerve Crush Injury Murine Model to Study Retinal Ganglion Cell Survival. J Vis Exp, 50.
Williams, D. (2007). Rabbit and rodent ophthalmic. EJCAP, 17(3).
Yin, X.-T., Tajfirouz, D. A., & Stuart, P. M. (2014). Murine Corneal Transplantation: A Model to Study the Most Common Form of Solid Organ Transplantation. J Vis Exp, 93.
Zor, F., & Karagoz, H. (2015). Composite Orbital and Periorbital Allotransplantation Model. In M. Z. Siemionow, Plastic and Reconstructive Surgery (pp. 369-372). Chicago: Springer.ç
| SKU | Description | Quantity |
|---|---|---|
| RWD-S12005-10 | IRIS-Fine fine cut-straight / pointed & pointed/10.5cm | 1 |
| RWD-S12004-09 | IRIS-Fine Fine Cut-Bend/Pointed&Pointed/9.5cm | 1 |
| RWD-F31047-12 | OLSEN-HEGAR Needle Holder (Cut)-Straight/2.15mm Width/12cm | 1 |
| RWD-F11001-11 | Fine tweezers-straight/tip 0.2*0.12mm/11cm | 1 |
| RWD-R31005-04 | Stainless steel micro-vascular clamp-straight/4*0.75mm/16mm | 5 |
| RWD-R34001-14 | Vascular clamp holder-with stainless steel micro-vascular clamp/14cm | 1 |
| RWD-S11001-08 | VANNAS Spring Shear-Straight/Mitsubishi/Pointed&Pointed/8cm | 1 |
| RWD-S11002-08 | VANNAS spring shear-bent/mitsubishi/pointed&pointed/8cm | 1 |
| RWD-F22002-10 | HARTMAN mosquito hemostatic forceps-straight / 0.8mm wide / 10.5cm | 1 |
| RWD-F22003-10 | HARTMAN Mosquito Hemostat-Curved/1mm Width/10cm | 1 |
| RWD-F35401-50 | Non-absorbent polyester suture (with needle) -3/8 round needle / 5-0 (50/box) | 0.2 |
| RWD-S32003-12 | Scalpel handle 3# (with ruler) -12.5cm | 1 |
| RWD-S31011-01 | Surgical blade-11# (box x 100 pieces/box) | 1 |
| RWD-SP0000-P | Surgical instrument bag-32*22cm | 1 |
| Weight | 4.41 lbs |
|---|---|
| Brand | RWD |
| Species | Rodent |
You must be logged in to post a review.
There are no questions yet. Be the first to ask a question about this product.
Monday – Friday
9 AM – 5 PM EST
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.
Reviews
There are no reviews yet.