$849.00 – $889.00
Cardiovascular surgery kits focus on the heart and blood vessels. Cardiac surgery kit is mainly used to treat the complexities of ischemic heart diseases, congenital heart diseases, valvular heart diseases, endocarditis, rheumatic heart diseases, and atherosclerosis.

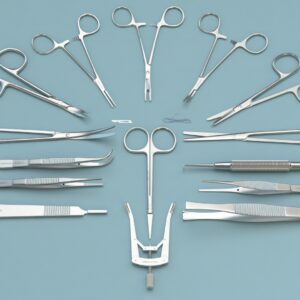
| Operating Scissors (Round Type) | S/S Str/11.5cm |
| IRIS-Fine Scissors (Round Type) | S/S Str/9.5cm |
| Spring Scissors (Triangular) | S/S Str/5*0.1mm/8.5cm |
| IRIS Dissecting Forceps-Large | Cvd, 0.8mm Tips, 10cm |
| Dressing Forceps | Str, 1.8mm Tips, 9cm |
| HARTMAN Mosquito Forceps | Str, 1.0mm Tips, 10cm |
| HARTMAN Mosquito Forceps | Cvd, 1.0mm Tips, 10cm |
| PGA Sutures w/Needle | o1/2/4×10/90cm/5-0 (50/Box |
| Sutures w/Needle | △3/8/2.5×7/30cm/6-0 (50/Box) |
| STEVENS Hooks, 1 Angled Tooth | (5mm long), 12.5cm |
| 3×3 Teeth Retractors-Blunt | 4.5cm |
| OLSEN-HEGAR Needle Holders with Scissors | Str, 12cm |
| SS Micro Clamps | Str/L*W 4*0.75mm/13mm |
| Clip Applicator for R31005- and R31006-Clamps | 14cm |
| Spinal Cord Hook | Tip Dia. 3mm/12cm |
| Instrument Storage Portfolio | 32*22cm |
| Operating Scissors (Round Type) | S/S Str/12.5cm |
| IRIS-Fine Scissors (Round Type) | S/S Str/10.5cm |
| Spring Scissors (Triangular) | S/S Str/5*0.1mm/8.5cm |
| IRIS Dissecting Forceps-Large | Cvd, 0.8mm Tips, 10cm |
| IRIS Dissecting Forceps | Str, 0.8mm Tips, 10cm |
| HALSTED Mosquito Forceps | Str, 1.0mm Tips, 12.5cm |
| HALSTED Mosquito Forceps | Cvd, 1.0mm Tips, 12.5cm |
| PGA Sutures w/Needle | o1/2/4×10/90cm/5-0 (50/Box) |
| Sutures w/Needle | △3/8/2.5×7/30cm/6-0 (50/Box) |
| STEVENS Hooks, 1 Angled Tooth (5mm long) | 12.5cm |
| 3×3 Teeth Retractors-Blunt | 4.5cm |
| OLSEN-HEGAR Needle Holders with Scissors | Str, 12cm |
| SS Micro Clamps | Str/L*W 4*0.75mm/13mm |
| Spinal Cord Hook | Tip Dia. 3mm/12cm |
| Clip Applicator for R31005- and R31006-Clamps | 14cm |
| Instrument Storage Portfolio | 32*22cm |
Cardiovascular surgery is mainly performed to treat the complexities of ischemic heart diseases, congenital heart diseases, valvular heart diseases, endocarditis, rheumatic heart diseases, and atherosclerosis. It also includes heart transplantation.
The preferred rodent species for conducting cardiovascular research is shifting from rat to mouse. The main advantage of using the rat is its bigger size. However, the development of more sophisticated micro-dissecting microscopes and imaging devices, high-caliber microsurgical instruments, small catheters for hemodynamic measurements, etc., has made microsurgery in the mouse more feasible than rats. Mouse models mimicking human diseases are essential tools in biomedical research, which aim to understand the underlying mechanisms of many disease states. Mouse models have gained popularity because of their small size, rapid gestation age (21 days), relatively low husbandry costs, and convenience in housing and handling. Moreover, the extensively characterized mouse genome and gene-targeting (i.e., knockout) and transgenic overexpression experiments are widely performed using mice rather rats (Tarnavski, 2009).
Thoroughly clean and sterilize the instruments used for the surgery. Autoclave and disinfect the equipment. Clear the operating area of any disturbances and ensure asepsis. Before the surgery, record the subject identification details such as strain and gender, most importantly note the weight of the subject. Also, examine the subject physically to assess its health status and activeness. Ensure that the subject has been acclimated to the facility appropriately. The acclimation process can last from a few days to a couple of weeks.
Anesthesia
Mouse Cardiac Surgery Protocol (Tarnavski et al., 2004)
Aortic Banding (Pressure-overload Model) Method (Tarnavski, 2009)
The steps mentioned above remain the same for both ascending aortic constriction and transverse aortic constriction.
Ascending Aortic Constriction (AAC)
Transverse Aortic Constriction (TAC)
Pulmonary Artery Banding (Right Ventricular Pressure-overload Model) Method
The principle of pulmonary artery banding is similar to that of aortic banding. However, while performing this surgery, be careful of specific challenges.
Myocardial Infarction Model (Permanent Ligation of the Artery) Protocol
Ischemia-Reperfusion Model Protocol
Coronary Artery Ligation and Intra-myocardial Injection in a Murine Model of Infarction Protocol (Virag & Lust, 2011)
Postoperative Management
Genomic Study of Coronary Diseases in Murine Models
Mouse models mimicking human cardiac diseases are essential paradigms to explain the underlying mechanisms of many disease states. Several surgical models have been developed that mimic human myocardial infarction (MI) and pressure-overload-induced cardiac hypertrophy. Microarray technologies, used in the research, measure the expression of thousands of genes simultaneously, allowing for the identification of genes and pathways that may potentially be involved in the disease process. In the study, a description of three major surgical procedures has been discussed: 1) aortic constriction, 2) pulmonary artery banding, 3) MI (including ischemia-reperfusion). The cardiac surgery techniques mentioned have been and will continue to be, essential for elucidating the molecular mechanisms of cardiac hypertrophy and genome profiling.
Evaluation of Galectin-1: a Biomarker of Surgical Stress in Murine Model of Cardiac Surgery (Hashmi & Al-Salam, 2015)
Galectin-1 (GAL-1) is a member of the β-galactoside-binding lectins family. GAL-1 regulates cell-cell and cell-matrix interactions, the immune response, apoptosis, cell cycle, RNA splicing, and neoplastic transformation. Satwat and Suhail investigated the effect of heart manipulation secondary to cardiac surgery on the level of GAL-1 in murine heart and plasma. They used male C57B6/J mice for the adopted model of cardiac surgery. Then the heart samples were processed for immunohistochemical and immunofluorescent labeling; Enzyme-linked immunosorbent assay and quantitative RT-PCR to identify GAL-1 levels in the heart and plasma during the first 24 hours following cardiac surgery. Results suggested that the GAL-1 is a valuable biomarker of surgical stress.
Evaluating Coronary Artery Ligation and Intramyocardial Injection in a Murine Model of Infarction
Mouse models are widely used to study acute injury and chronic remodeling of the myocardium in vivo. With the advancement of genetic modifications to the whole organism or the myocardium and an array of biological and synthetic materials, there is an excellent potential for any combination of these to mitigate the extent of the acute ischemic injury and impede the onset of heart failure according to myocardial remodeling.
The study presented the methods and materials used to perform the microsurgery reliably and the modifications for temporary (with reperfusion) or permanent coronary artery occlusion studies as well as intramyocardial injections. Development of miniature technology for imaging in vivo, analyzing large-scale genomics and proteomics, drug screening, efficacy of cell-based and/or protein therapies as well as biomaterials, combined with the increasingly wide range of genetic manipulations afforded by ubiquitous or tissue-specific transgenic or mutant/knockout mice, the murine model of myocardial infarction, undoubtedly, continue to be an invaluable tool in evaluating acute cardiac injury and long-term remodeling. Therefore, there is an absolute value in being able to perform these experiments reliably and reproducibly.
Murine cardiac surgical procedures are relatively short, so it is not necessary to withhold food and water from mice before the surgery. Before starting the surgical procedure, ensure the adequate depth of the anesthesia using a toe pinch test or other anesthesia assessment tests. It is important not to disturb or agitate the animal while the anesthetic is taking effect to ensure smooth induction and facilitate subsequent procedures. Do not administer pre-surgical analgesia (buprenorphine) since narcotic analgesics are known to depress the respiratory center and may interfere with the survival after the open-chest surgery. Since the described surgical procedures are relatively short (15–20 min), it is not critical to strictly regulate the core temperature of the mouse. Do not pull apart the pericardial sac with too much force as it may rupture the wall of the left superior vena cava and cause bleeding that can be fatal. The significant challenges that arise during the cardiac surgery are because of the extremely thin and delicate walls of the pulmonary trunk and the incapacity of the right ventricle to undergo stress while the pulmonary artery is being manipulated. Carry out the whole surgical procedure under aseptic conditions.
The most widely used rodent species for conducting cardiovascular research is the mouse. The significant advantage of using the rat is its bigger size. However, the development of micro-dissecting microscopes and other imaging devices, high-caliber microsurgical instruments, small catheters for hemodynamic measurements, etc., has made microsurgery in the mouse as feasible as in the rat. Mouse models have gained popularity because of their small size, rapid gestation age (21 days), relatively low maintenance costs, convenience in housing and handling, and fewer compound requirements for pharmacological studies. Use of a simple dissecting scope or magnifying glass and well-lit conditions enable the vasculature to be seen readily. To exterminate the risk of postoperative mortality, it is essential to avoid severing large vessels since the total blood volume of a 25g mouse is less than 2ml. If excessive bleeding occurs, gentle application of pressure or pinpoint cauterization can be used to stop the bleeding. Also, the mouse genome has been extensively characterized, and gene-targeted (i.e., knockout) and transgenic overexpression experiments are more commonly performed using mice rather than rats.
| Weight | 4.41 lbs |
|---|---|
| species | Rat |
You must be logged in to post a review.
There are no questions yet. Be the first to ask a question about this product.
Monday – Friday
9 AM – 5 PM EST
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.
Reviews
There are no reviews yet.