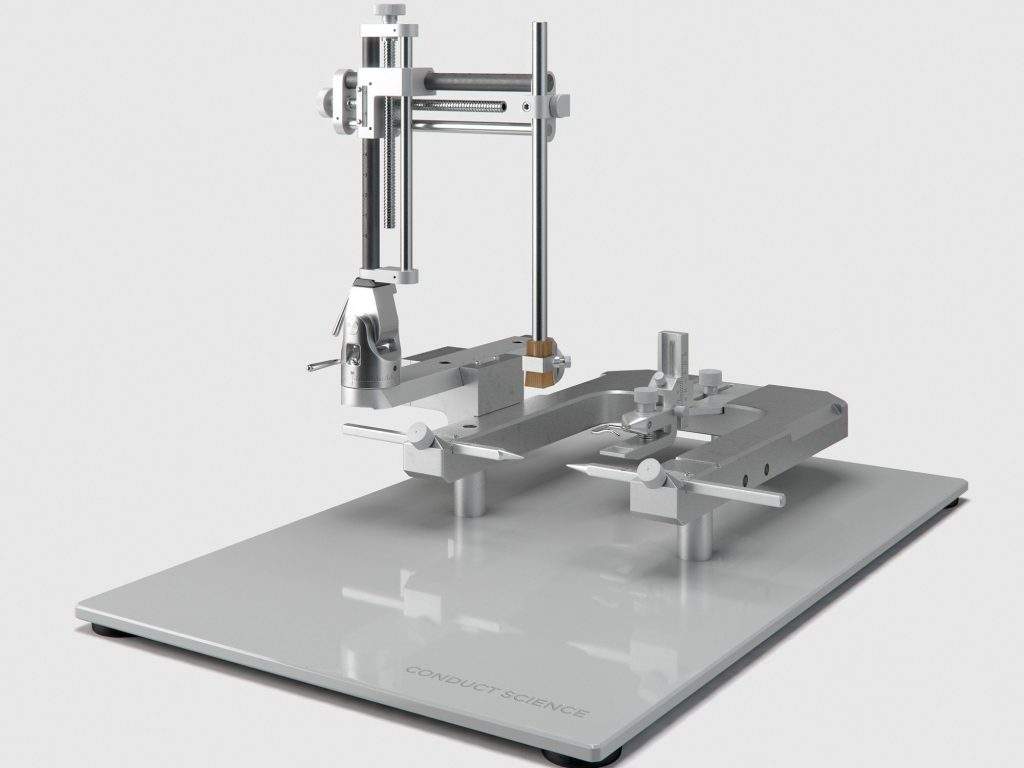
Our Stereotaxic Categories
-
 $220.00 – $384.00Select options This product has multiple variants. The options may be chosen on the product page
$220.00 – $384.00Select options This product has multiple variants. The options may be chosen on the product page -
 $180.00 – $495.00Select options This product has multiple variants. The options may be chosen on the product page
$180.00 – $495.00Select options This product has multiple variants. The options may be chosen on the product page -
 $360.00 – $461.00Select options This product has multiple variants. The options may be chosen on the product page
$360.00 – $461.00Select options This product has multiple variants. The options may be chosen on the product page
Stereotaxic Products
-
 $13,790.00 – $13,990.00Select options This product has multiple variants. The options may be chosen on the product page
$13,790.00 – $13,990.00Select options This product has multiple variants. The options may be chosen on the product page -
 $1,034.00Select options This product has multiple variants. The options may be chosen on the product page
$1,034.00Select options This product has multiple variants. The options may be chosen on the product page -
 $136.00Select options This product has multiple variants. The options may be chosen on the product page
$136.00Select options This product has multiple variants. The options may be chosen on the product page -
 $1,033.00Select options This product has multiple variants. The options may be chosen on the product page
$1,033.00Select options This product has multiple variants. The options may be chosen on the product page -
 $0.00 – $8,579.00Select options This product has multiple variants. The options may be chosen on the product page
$0.00 – $8,579.00Select options This product has multiple variants. The options may be chosen on the product page
Documentation
Introduction
Stereotaxic surgery, also known as stereotactic surgery, is a three-dimensional technique used to locate and treat deep tissue lesions using heat, chemicals, or cold. The use of stereotaxic surgery to implant microdialysis probes, guide cannulas in animals, and stimulate electrodes in specific areas of the central nervous system (CNS) plays a crucial role in understanding neurobiology related to reward and aversion. The principles of stereotaxic surgery were described by the British scientists Horsley and Clarke, who developed the first stereotaxic instrument in 1908. Stereotaxic procedures like craniotomy drilling, electrode implantation, and dura matter removal in animal models play a pivotal role in neuroscience research conducted for behavioral analysis.
Stereotaxic Apparatus
In a stereotaxic apparatus, a U-shaped stereotaxic frame is mounted on a base plate. Three microdrives (or the mechanical elements) are fixed on the stereotaxic frame to facilitate the movement of the cannula/electrode holder along three orthogonal axes, including forward and backward, up and down, and side to side. A micromanipulator assembly on the frame’s side can be mobilized in three dimensions by three micrometric vernier screw drives. The animal is held in place with the help of two lateral ear bars and an adjustable incisor bar.
The microdrives facilitate movement in 3D spaces, with the help of three vernier scales, thus enabling movement along the anteroposterior (AP) ‘y’ axis, which describes a line moving from anterior to the posterior part of an animal’s head, the mediolateral (ML) ‘x’ axis running from midline to left or right side of the head, and a dorso-ventral (DV) ‘z’ axis running from skull’s surface to the interior of the brain. The travel amplitude of three micromanipulators is typically 80nm, each consisting of a graduated mobile arm that moves along the apparatus. Conduct Science offers a wide range of stereotaxic instruments according to your experimental needs.
Determination of Stereotaxic Coordinates
In a stereotaxic apparatus, a U-shaped stereotaxic frame is mounted on a base plate. Three microdrives (or the mechanical elements) are fixed on the stereotaxic frame to facilitate the movement of the cannula/electrode holder along three orthogonal axes, including forward and backward, up and down, and side to side. A micromanipulator assembly on the frame’s side can be mobilized in three dimensions by three micrometric vernier screw drives. The animal is held in place with the help of two lateral ear bars and an adjustable incisor bar.
The microdrives facilitate movement in 3D spaces, with the help of three vernier scales, thus enabling movement along the anteroposterior (AP) ‘y’ axis, which describes a line moving from anterior to the posterior part of an animal’s head, the mediolateral (ML) ‘x’ axis running from midline to left or right side of the head, and a dorso-ventral (DV) ‘z’ axis running from skull’s surface to the interior of the brain. The travel amplitude of three micromanipulators is typically 80nm, each consisting of a graduated mobile arm that moves along the apparatus. Conduct Science offers a wide range of stereotaxic instruments according to your experimental needs.
Protocol of Implanting Guide Cannula in Rodent’s Brain
The experimenter can implant a guide cannula or microdialysis probe by following the underlying procedure (Poole et al., 2019) (Geiger et al., 2021).
- Take a rodent from its home cage and immobilize it with a nose cone on a stereotaxic apparatus.
- To anesthetize the subject, turn on the isoflurane machine at a flow rate of 0.8L/min.
- Confirm sedation by using “pinch withdrawal reflex.”
- Use a heating pad to keep the animal thermoregulated throughout the surgery and monitor the temperature with a rectal thermometer.
- After sedating the animal, shave its fur and sterilized the exposed skin with iodine solution.
- Pass one ear bar through one ear canal and the other ear bar through the other ear to fix the animal on the stereotaxic apparatus. Position the animal properly and completely immobilize it.
- Now, use the incisor bar to secure the subject’s mouth. The head should be leveled. To ensure that the head is leveled, take a ruler, and hold it in a position vertical with respect to the stereotaxic frame. The angle between the middle of the animal’s scalp and the ruler should be 90o. Some stereotaxic instruments digitally confirm this positioning.
- Use a sterile scalpel to make an incision, starting at the back of the eyes and moving towards the lambda. This anteroposterior incision should be 2cm.
- Expose the incision using hemostats and have a clear view of the skull.
- Now, use a cotton swab to remove pericranial tissues and place the guide cannula so that it is positioned at the bregma. Place the probe into the rodent’s nucleus accumbens such that stereotaxic coordinates are 10mm anterior to zero, 1.2mm lateral to midsagittal sinus, and 4mm ventral to level skull surface.
- Add or subtract from bregma coordinates to precisely position the cannula/probe in the desired coordinate within the skull. The drilling will take place at this ventral coordinate.
- Use a drill to make a small hole through the skull and ensure that this hole is large enough to allow the insertion of the cannula without it touching the sides of the hole.
- Slightly crack up the meninges using a sterile needle. Make six holes, two anterior, two posterior, and two lateral holes. Tighten the sterilized screws into the skull.
- Follow the surgical protocol to place and secure the cannula properly. Cover the exposed skull surface with liquid dental cement.
- Take away the hemostats as the cement dries up.
Probe Placement Confirmation
Once the probe is implanted, the researcher must verify the accurate placement of the probe at the target site. This confirmation is significant when the subject’s age, gender, or strain is different from those used to derive stereotaxic atlases. The following procedure is used for probe placement verification.
- Anesthetize the animal and perfuse its brain with saline and paraformaldehyde via the heart.
- Remove the skull cap with cannulas or probes and extract the brain.
- Cut at the location of the hole from where the probe was inserted.
- Perform histological examination to identify probe location.
Cerebral Microdialysis
Microdialysis is an invasive surgical method to access the cranial tissues’ extracellular space. Microdialysis probes are inserted in anesthetized animals’ brains for their behavioral analysis. However, the experiments can also be performed in awake and freely moving animals. It is a sampling technique, not a measuring technique. Thus, the substance under study must be quantified from dialysate using the available high-sensitivity analytical methods (Pierce et al., 2020).
The microdialysis procedure mimics the passive function of a capillary blood vessel in which molecules show passive diffusion across the concentration gradient from an area of higher concentration to lower concentration. This technique can be used to collect or deliver the sample to the extracellular space in a process called “retro dialysis.” The dialysate can contain neurotransmitters, metabolites, metabolic precursors, or waste products. Additionally, the length of the dialysis membrane varies from 0.5-2mm. The animal is anesthetized before stereotaxic surgery, and inhalable anesthetics are preferred for this purpose as the animal can rapidly recover from their effects. These anesthetics can alter physiological parameters. For example, isoflurane administration can increase the lactate levels in the mouse brain several-fold. Injectable anesthesia is used for implanting guide cannulas, and surgery starts many days later to minimize the effect of these anesthetics. The flow rate through the probe ranges from 0.5 to 3µl/min.
The microdialysis method is stereotaxically used to measure the level of neurotransmitters and energy metabolites. For instance, to study the effect of dopamine-releasing drugs on the subjects’ midbrain’s dopamine projections. These drugs are introduced via microdialysis probes to work precisely on specific sites. Stereotaxic surgery to implant probes or cannulas is required before the microdialysis procedure.
Microdialysis Protocol (Koeing et al., 2018)
- Once the stereotaxic surgery is complete, perfuse artificial cerebrospinal fluid (aCSF) through the porous probe. The analytes from the brain’s extracellular space will diffuse into the aCSF.
- Use two small pieces of plastic tubing to push the artificial cerebrospinal fluid through the probe and collect it post-dialysis.
- Attach an inflow tubing to a syringe pump to push aCSF through the probe at the rate of <2.5µl/min.
- Connect the outflow tubing to a microfuge tube for collecting dialyzed aCSF.
- Attach both inflowing and outflowing tubing to a 12 inches long lightweight metal wire.
- Attach the metal wire to the tether screw at one end and a liquid swivel at the other.
- Hang the swivel on the arm in the center of the cage forming a swivel-wire-arm assembly. This assembly facilitates animal’s free movement across the cage without getting tangled in the probe.
- Habituate and equilibrate the animals for 8-10hours before sample collection.
- Collect the samples after the 6-8hours interval. The animals spend a total of 14-18hours in the cage equipped with inflow and outflow tubing without any damage.
- Provide the animals with food and water ad libitum.
Strengths and Limitations
The use of stereotaxic surgery to implant guide cannulas in microdialysis procedures has proven advantageous in several ways. One advantage is that the probe can be inserted multiple times over several weeks for behavioral analysis. It is also beneficial when the experiment requires low-stress animals to study locomotor activity and diurnal rhythm. Such surgeries are detectable for up to several days. Moreover, several analytes or neurotransmitters can be simultaneously sampled by microdialysis.
On the other hand, the invasive nature of surgery can damage implantation areas in the brain. Probe implantation initially impairs the brain-blood barrier, decreasing blood flow in the region, thus causing an abnormal neurotransmitter release. A reduction in glucose and lactate levels is also observed. However, these issues appear to resolve 18-24 hours following implantation. Therefore, the experiments must be conducted one to two days after probe implantation.
Summary
- Stereotaxic surgery, also known as stereotactic surgery, is a three-dimensional technique used to locate and treat deep tissue lesions using heat, chemicals, or cold.
- Stereotaxic surgery is used for microdialysis probes or guide cannula implantation in rodent models for behavioral and neurobiology analysis.
- In a stereotaxic apparatus, a U-shaped stereotaxic frame is mounted on a base plate.
- The stereotaxic frame comprises three axes, anteroposterior, dorsoventral, and mediolateral, used to immobilize the animal properly within th stereotaxic frame.
- Microdialysis is an invasive surgical method to access the cranial tissues’ extracellular space.
- The microdialysis procedure mimics the passive function of a capillary blood vessel in which molecules show passive diffusion across the concentration gradient from an area of higher concentration to lower concentration.
- Stereotaxic surgery presents many advantages for neurobiological analysis, yet its invasive nature makes it painful for the subjects.
References
- Koenig, M., Thinnes, A., & Klein, J. (2018). Microdialysis and its use in behavioural studies: Focus on acetylcholine. Journal of neuroscience methods, 300, 206-215.
- Poole, E. I., McGavin, J. J., Cochkanoff, N. L., & Crosby, K. M. (2019). Stereotaxic surgery for implantation of guide cannulas for microinjection into the dorsomedial hypothalamus in young rats. MethodsX, 6, 1652.
- Geiger, B. M., Irene, M., & Pothos, E. N. (2021). Stereotaxic Surgery in Rodents for Stimulation of the Brain Reward System. In The Brain Reward System (pp. 21-50). Humana, New York, NY.
- Pierce, C. F., Kwasnicki, A., Lakka, S. S., & Engelhard, H. H. (2020). Cerebral Microdialysis as a Tool for Assessing the Delivery of Chemotherapy in Brain Tumor Patients. World Neurosurgery.
Let's work together!
Have questions? Ask anything!