
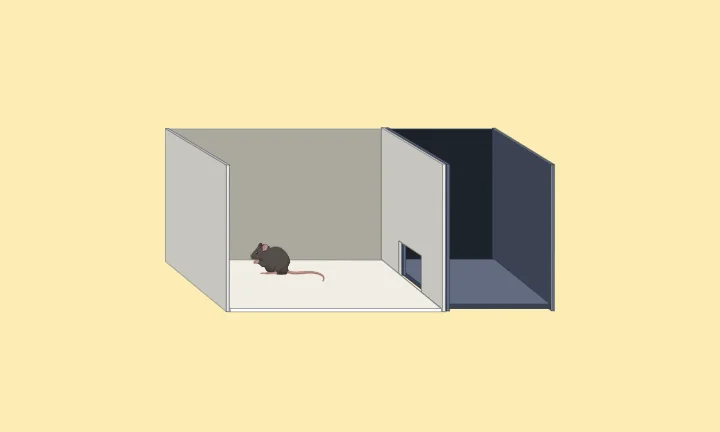
The light/dark box paradigm, first introduced by Crawley and Goodwin in 1980, is a behavioral test designed to evaluate anxiety-like behavior and stress responses in rodents [1]. This test capitalizes on the natural aversion of rodents to brightly illuminated, open spaces, which they perceive as potentially dangerous, contrasting with their preference for dark, enclosed areas offering a sense of safety and security [2].
The light/dark box is divided into two distinct chambers. One chamber is small and dark, taking up one-third of the total space. The other chamber is larger and brightly lit, occupying the remaining two-thirds. A small tunnel connects the two chambers, allowing the subject animal to move freely between the dark and illuminated areas.
The light/dark box is mainly used to study stress and anxiety and to test the effectiveness of drugs that reduce these conditions [3]. Stressful situations, like mild brain injuries or anxiety-inducing drugs, make animals spend less time in the lit area. This behavior can be reversed with common anti-anxiety medications like benzodiazepines [4]. The light/dark box is a reliable tool for testing treatments that aim to reduce stress and anxiety in rodents, regardless of the type of anxiety-like behaviors induced [5].
However, the test conditions of the light/dark box are naturally anxiety-inducing, so additional stress methods (behavioral or pharmacological) are not necessary to evaluate the effectiveness of antidepressant and anxiolytic drugs. For example, commonly used antidepressants like moclobemide and selegiline were tested in the light/dark box during their early development [6]. Therefore, whether or not anxiety-like behaviors are induced, the light/dark box offers a suitable environment for testing therapies aimed at reducing stress and anxiety in rodents.
Figure: Light Dark Box
To conduct a test in the light/dark box, let the rodents get used to the environment for at least one hour before the test. You can clean the box between animals for hygiene or leave it “soiled” to avoid new smells that might stress the animals [3].
During the test, the rodent is placed in the illuminated compartment facing away from the connecting tunnel, allowing free movement between the two chambers. The duration of the test typically ranges from 5 to 10 minutes, during which multiple parameters such as time spent in each compartment and exploratory behavior are recorded [3].
Animals should not be familiarized with the light/dark box before testing because the test relies on novelty and exploratory behavior. Prior exposure to the light/dark box can negate the effects of anxiolytic and anxiogenic drugs, as the animals would already be comfortable with the environment and wouldn’t see a significant safety difference between the two compartments. Therefore, animals should only be tested in the light/dark box once, making it suitable for between-group comparisons but not within-group comparisons.
Figure: The Light/Dark Box Test [13].
Common measurements in the light/dark box include the time spent in the light compartment versus the dark compartment and the number of crossings between the two compartments. The former parameter is often considered indicative of anxiety levels, with decreased time in the lit area suggesting higher anxiety [3]. Additionally, the “latency to first crossing” metric, reflecting the time taken for the first transition between compartments, provides insights into initial exploratory behavior and potential differences between experimental conditions [4].
Rodent strain and sex may influence outcomes in the light/dark box test. Variations in baseline anxiety levels have been observed between different strains of mice and rats, impacting their responses to pharmacological interventions [7]. Moreover, sex-specific differences in anxiety-related behaviors have been reported, emphasizing the importance of considering the sex of experimental animals in study design and data interpretation [8].
For example, studies have shown baseline differences between two common mouse strains, BALB/c and C57BL/6j. BALB/cj mice exhibit higher baseline anxiety levels compared to C57BL/6j mice. Consequently, BALB/cj mice are more sensitive to the anxiolytic effects of diazepam [9]. This is important for researchers who use multiple mouse strains in their experiments.
Similarly, comparing Sprague Dawley and Dark Agouti rats reveals baseline differences in light/dark box performance. Dark Agouti rats spend less time in the open, lit area compared to Sprague Dawley rats [10]. Although both strains respond to diazepam by spending more time in the lit area, these baseline differences are crucial for researchers to consider when optimizing their experiments.
There is also ongoing debate about whether male and female rats behave differently in the light/dark box. For example, one study found that female rats showed less baseline anxiety than males in stressful tests like the elevated plus maze and open field maze, but no difference was observed between the sexes in the light/dark box [11]. Conversely, other studies found that female rats spend more time on the light side of the box, indicating lower baseline anxiety levels [12]. Additionally, in one study, the anxiogenic drug scopolamine significantly reduced the time females spent in the light compartment, suggesting they might be more sensitive to certain anxiety-inducing conditions. Therefore, researchers should consider the sex of their animals to identify any potential differences.
The light/dark box test serves as a straightforward and reliable method for evaluating anxiety and stress-related behaviors in rodents. Its minimal training requirements and versatility make it suitable for assessing a wide range of experimental conditions, with potential variations between rodent strains and sexes warranting careful consideration in experimental design and result interpretation. Overall, the light/dark box remains a valuable tool in preclinical research for investigating anxiety disorders and evaluating therapeutic interventions.

Vanja works as the Social Media and Academic Program Manager at Conduct Science. With a Bachelor's degree in Molecular Biology and Physiology and a Master's degree in Human Molecular Biology, Vanja is dedicated to sharing scientific knowledge on social media platforms. Additionally, Vanja provides direct support to the editorial board at Conduct Science Academic Publishing House.
