
Medical advancements are an essential requirement in today’s world to reduce the financial burden of diseases and safeguard the healthcare systems. For diseases like cancer, better drug delivery approaches, diagnostic methods, and treatment options are required to save more patients and enhance their quality of life.
One such approach developed — being tested, and applied in some disease cases — is the use of liposomes to enhance drug delivery in organisms. And besides drug delivery, they also have applications in analytical biochemistry and medical diagnostics.
Liposomes are little bubbles (vesicles) made of the same substances as cell membranes. They get their names from two Greek words: ‘Lipos,’ which means fat, and ‘Soma,’ meaning body.[1]
They were discovered in 1964 by a British hematologist Dr. Alec D Bangham, and another scientist, R. W. Horne. They were discovered by applying negative stains to dry phospholipids when the two scientists tested the institute’s new electron microscope.[1]
In this article, you will learn more about liposomes: what they are, their structural composition, how they are formed, their classification, properties, advantages and disadvantages, and their applications in biomedicine/pharma to develop better diagnostic and treatment approaches.
Liposomes are circular-shaped vesicles formed by one or more biological bilayer membranes that separate aqueous fluids.[2] They have two main parts: hydrophilic polar head groups that can either be zwitterionic (having both positive and negative charges) or negatively charged; and hydrophobic hydrocarbon chains with different chain lengths and degrees of unsaturation.[2]
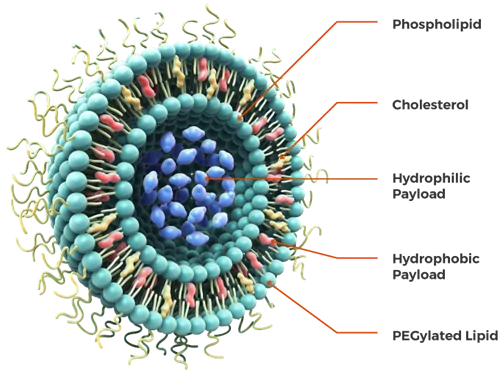
Figure: An illustration of liposome and its structural components.[3]
Liposomes structurally consist of cholesterol, and non-toxic phospholipids, explained below in detail.[2]
Cholesterol alone can’t form the liposome structure. However, with phospholipids, cholesterol can be incorporated into liposomes, improving the stability of the vesicle and preventing the aggregation of molecules. It’s also an essential factor involved in the regulation of the fluidity of liposomes.[4]

Figure: An illustration of the preparation of liposomes and cholesterol roles in its stability.[5]
Besides phospholipids and cholesterol, some other additives are added while preparing synthetic or semisynthetic liposomes.
The presence of polyethylene glycol (PEG) on the surface of the liposomes can protect the metabolic inactivation and degradation of combined drugs, increase the circulation time of liposomes systemic circulation, and enhance the intracellular uptake of liposome vesicles.[4]
For the preparation of charged liposomes, charged phospholipids, like stearyl amine (SA) and dicetyl phosphate (DCP), are used.[4]
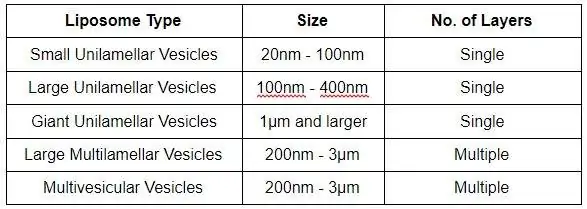
Liposomes are versatile molecules and can be classified in several ways based on their diversity and structural properties, such as composition, shape, size, and surface properties.

Figure: An illustration of unilamellar and multilamellar vesicular structures.[6]
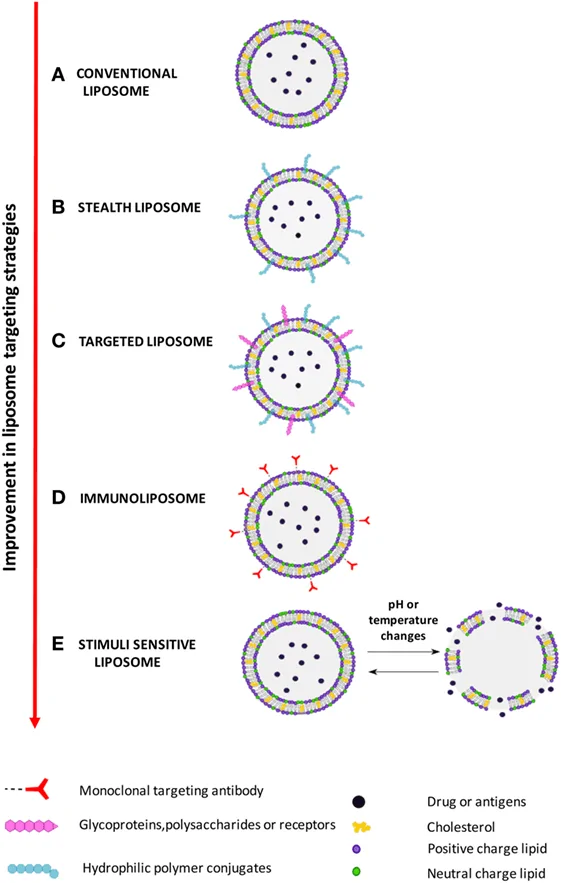
Figure: An illustration of different types of liposomes based on their composition and targeting strategies.[6]
Some of the properties of liposomes imparted due to their structural characterization and organization are:[1]
The main advantages of liposomes are considered over their use in drug formulation and delivery. Some of its advantages are:
Despite having all the advantages above, liposomes have some limitations that prevent their extensive uses in clinical and therapeutic areas. These include:
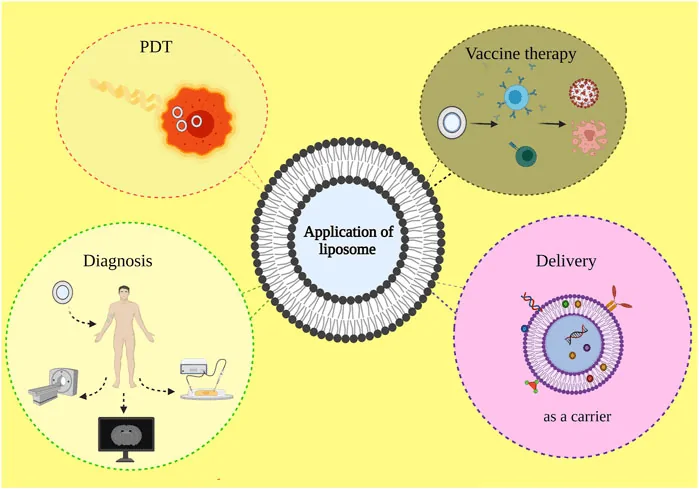
Figure: An illustration of different application areas of liposomes: photodynamic therapy, drug delivery, disease diagnosis, and vaccine therapy.[5]
Liposomes are small spherical vesicles consisting of different phospholipids, cholesterol, and other molecules. Their structural organizations make them perfect as carriers for transporting and delivering a wide range of drugs.
Thus, they have extensive applications in diverse areas, including disease diagnosis, drug and gene delivery, contrast agents for medical imaging, and model cell membrane. They offer several advantages over other available therapeutics, including site-specific-drug delivery, reduced toxicity, biocompatibility, improved efficiency, and many more.
Besides having multiple advantages and their use in several medical areas, liposomes are not without limitations. However, scientists worldwide are working to reduce these limitations, leading to their extensive use in diagnostic assays, immunotherapy, and targeted delivery of drugs.
Thus, emerging scientists hold a golden opportunity to unravel the potential of liposomes in clinical therapeutic applications, leading to better options of diagnosis and treatments for fatal diseases.
