
There are several microbiology techniques and procedures specially developed over the years to study and understand the metabolic processes, genetics, functions, and interaction of microbes with other organisms.[1]
The methodologies mostly involve techniques for culturing, identification, isolation, staining, and engineering these tiny organisms. They also have applications in other areas of biological research, including genetics, plant physiology, evolution, and molecular biology.[1]
Moreover, some of them contribute to better our health, but some cause life-threatening diseases. Others are used in food and beverage production, and to understand all this, we need to study these organisms.
Given the myriad uses of microbiology and its basic techniques, this article presents an overview of the basic microbiological laboratory techniques, how they work and are used in labs.
The microbiology techniques are categorized based on the type of experiments. It includes:[2]
These techniques don’t cover every microbiology technique; however, they are fundamental to all practices performed in microbiology laboratories.
It’s the complete removal of all other microbial forms, including viruses, bacteria, fungus, spores, and other vegetative cells from the surface or the culture media.
Based on the purpose of the sterilization, the method is categorized into two groups:
Disinfection is the process of killing microbes or inhibiting their growth from inanimate objects or surfaces by using physical or chemical agents like phenol, chlorine, alcohol, and heavy metal and their compounds.[2]
It’s the complete elimination of all pathogenic and non-pathogenic microbes from surface tops to reduce contamination. It’s also employed in daily lives to sanitize hands or in restaurants, dairies, and breweries to remove microbes and prevent infection and contamination.
It involves using chlorine-based cleaners, alcohol-based cleaners, formaldehyde, and hydrogen peroxide.[2]
Before introducing the microbial strain to the culture media, the isolation and inoculation techniques are followed.[3]
It’s a basic technique used in microbiology labs to place microbial cultures onto a culture medium. It’s performed using an apparatus, called inoculation loop, made of platinum or nichrome wire with a loop at its one end. It’s mainly used in streaking and culture plate techniques.[3] The small sample picked up and transferred from the culture is known as inoculum.
Isolation is a microbiological technique in which a specific microbial strain is isolated from a mixed culture of microorganisms by culturing the microbes on a selective culture media.[3] However, the procedure must be repeated several times to eliminate contamination by other microbes and achieve a pure culture of the microbial strain, which is then observed in culture plates as discrete/isolated individual colonies.
Microbes are grown in labs on culture media, which supply their nutritional requirements. These requirements vary for different microorganisms, thus a spectrum of culture medium recipes have been developed by scientists to obtain the desired microbial strain.[3]
Now, the common culture techniques used in microbiology labs include:[5]
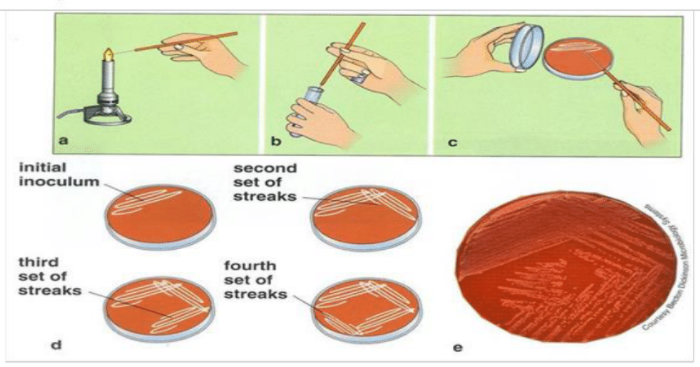
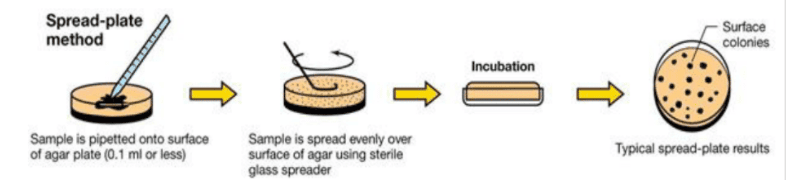
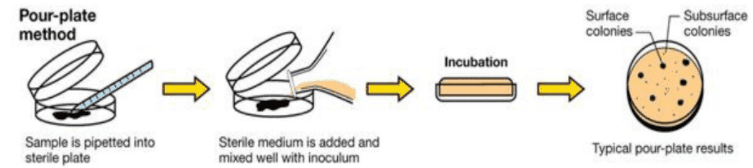
After the microorganisms are inoculated in plates, the culture plates are sealed from base to lid using adhesive tape to prevent contamination. Then, the plates are kept in the incubator for the required time and temperature for the growth of the organisms. Furthermore, keeping the plates in an inverted position prevents the formation and fall of water droplets into the culture media.[4]
If it’s required to store the microbial samples for later experiments, the following storage techniques are used:
Counting microbial colonies is an essential task in performing a range of experiments. Here are some enumerating techniques:[6]
Pathogen identification is important for several applications. For example, it’s used to know which microbe is involved in contamination and food spoilage, which has useful applications in human lives, and which microorganism caused the particular disease for correct diagnosis and treatment in hospitals.
Microbes are identified by:[5]
Microbiology techniques are required to study microorganisms’ structure, function, metabolism, and genomics. They help understand how microbes work, interact with living organisms, cause diseases, and how they can be applied for human use.
The fundamental microbiology laboratory techniques include aseptic techniques, culturing techniques, enumerating bacteria, and identifying different classes of microorganisms. These techniques form the base of advanced research and experiments performed on microorganisms. The data obtained through these experiments also relate to other branches of biology, including molecular biology, soil science, agriculture, and the evolution of organisms.
The microbial study has helped scientists in many ways, from understanding how life began on earth to the use of microbes to control pollution. Moreover, the efficient use of these microbiological tools provides a deeper understanding of microbial life and the discovery of new species with new mysteries.
