Mutarotation
Mutarotation is a change in the optical rotation of a solution due to a change in the equilibrium between alpha (ɑ) and beta (β) anomers, upon dissolution in the aqueous solution.The process is also known as anomerization.
The concept of mutarotation is related to the optical rotation and activity of the compounds dissolved in the solution. What are these terms? To better understand the concept of mutarotation, some of the frequently used terms are explained below.
What are anomers?
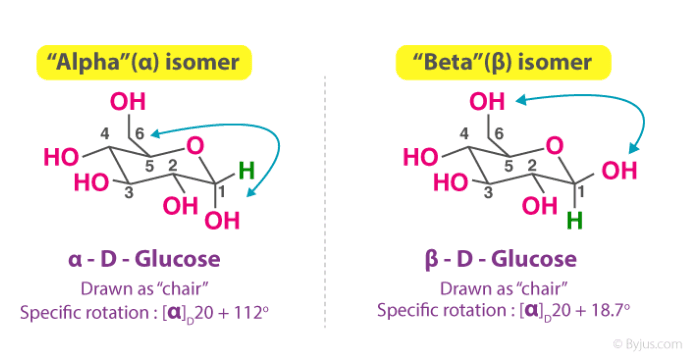
Anomers are epimers (diastereomers differing at one carbon) that differ from each other in the configuration of C1 if they are aldoses and in the configuration of C2 if they are ketoses.
What is optical rotation?
Optical rotation is the angle through which the plane of polarized light moves or rotates when a linearly polarized light travels through a layer of liquid or certain other materials.
What’s polarized light?
When light is transmitted through certain media, the vibrations of the light waves occur in a single plane. This is called the polarization of light.
What does equilibrium between anomers mean?
It’s a state of balance between two forms of a compound (alpha and beta) in a solution.
What is the optical activity?
It’s the ability of a compound to rotate the plane of polarized light. It occurs in the compounds possessing chirality (asymmetry or compounds which lack mirror symmetry).
The optical activity of chiral compounds is generated when the electromagnetic radiation of polarized light interacts with the asymmetric field of electrons of these compounds.
The compounds are known to be optically active when they rotate the linearly polarized light. And it must be noted that all optically active compounds have their own specific rotation.
History and Origin of The Concept of Mutarotation
The phenomenon of mutarotation was discovered in 1846 by Augustin Pierre Dubrunfaut. His whole study was based on sugars. He noticed that when sugar is dissolved in water, its optical activity changes in time.
From the value of 110°, it decreases to 52°. He named this phenomenon “birotation”. Later, in 1899, Lowry gave it a more appropriate name “mutarotation”, which signifies the concept behind it.
After this, several other scientists got involved in understanding the phenomenon of mutarotation. The series of discoveries answered many questions like “how does it occur? Why does it occur? Does it occur in other mediums as well? Is this phenomenon observed in other compounds as well? Is it observed in all kinds of sugar? If no, why not?” The answer to these questions is explained as a different subtopic in this article.
Given below is a series of events that occurred after the discovery of mutarotation, for an in-depth understanding of this phenomenon.
Mechanism of Mutarotation
The discovery of mutarotation, which began with glucose, had opened a wide area of research for scientists to study this phenomenon in other crystalline sugars.
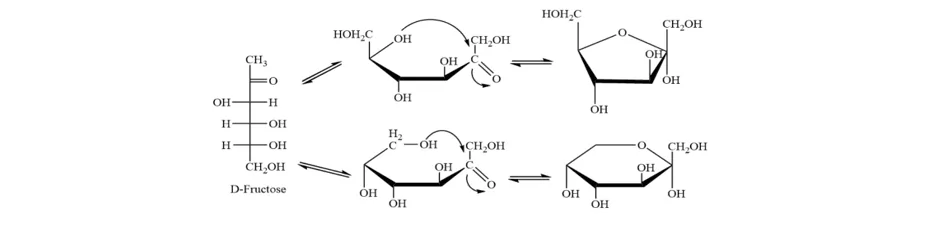
Later, it was found in lactose, galactose, arabinose, maltose, xylose, fructose, fucose, rhamnose, mannose, rhodeose, gentiobiose, melibiose, perseulose, and several rare synthetic sugars.
In conclusion, all reducing sugars undergo mutarotation in an aqueous solution and non-reducing sugars don’t possess this property.
What's a reducing sugar?
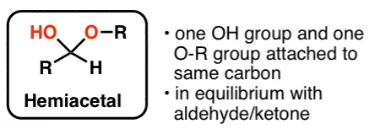
Any sugar that has a free aldehyde or ketone group is considered as reducing sugar. They are also called hemiacetal compounds. These sugars are in equilibrium with the open-ring form of the molecule.
They contain an aldehyde group which acts as a reducing agent towards certain metal salts. For example, they reduce copper ion (Cu2+) in Benedict’s test and Fehling solution and silver ion (Ag+) in Tollen’s test.
What is a non-reducing sugar and why don’t they show mutarotation?
Non-reducing sugars don’t have any free aldehyde or ketone groups. This is also the reason why they are not oxidized by a weak oxidizing agent or do not possess reducing power. Examples include sucrose and trehalose.
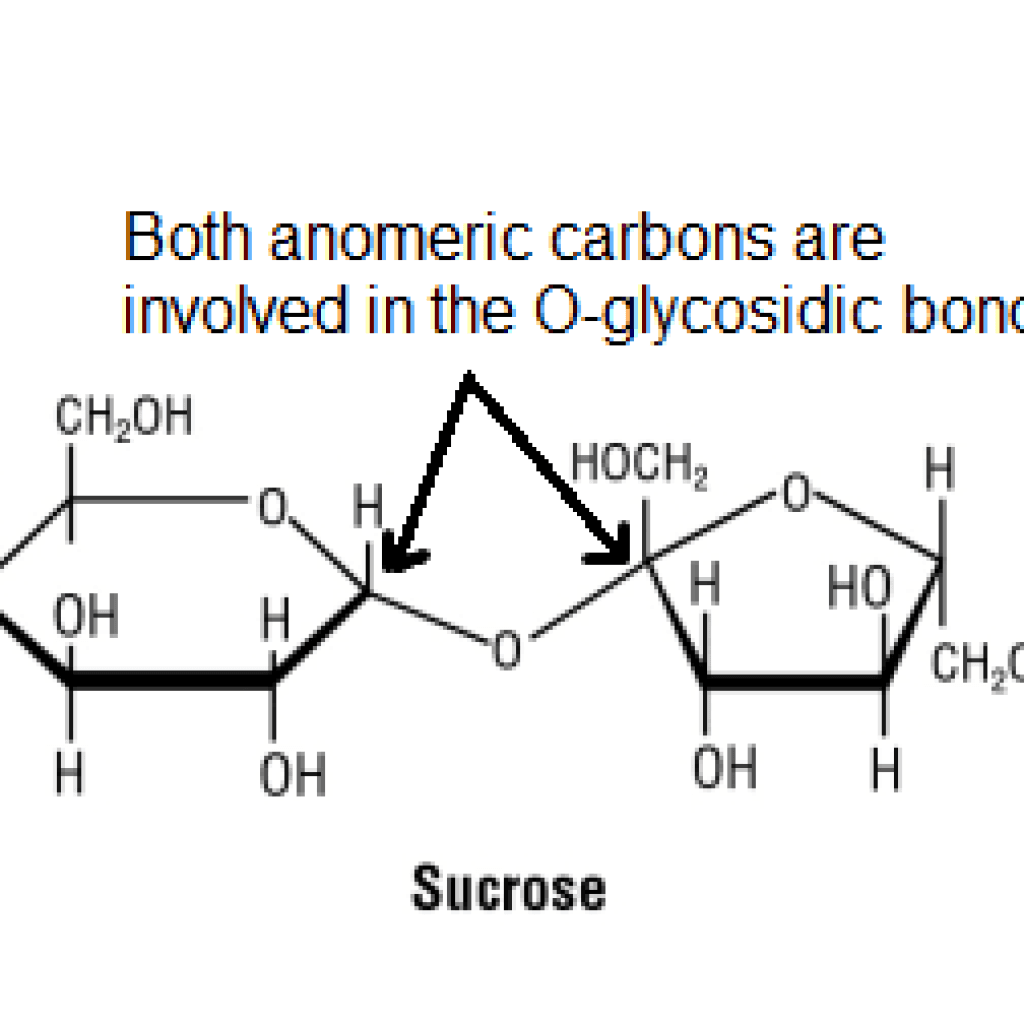
To understand the concept of mutarotation in non-reducing sugars, let’s take the example of sucrose. Sucrose is formed by a condensation reaction between a glucose molecule and a fructose molecule.
The condensation reaction involves the anomeric carbons of glucose and fructose that lead to the formation of an O-glycosidic bond between the two molecules. For mutarotation to occur, a compound must have a free-anomeric carbon.
But in sucrose, both the anomeric carbons are involved in the formation of glycosidic linkage, because of that, they don’t exhibit the phenomenon of mutarotation.
Non-reducing sugars don’t have any free aldehyde or ketone groups. This is also the reason why they are not oxidized by a weak oxidizing agent or do not possess reducing power. Examples include sucrose and trehalose.
The Occurrence and Measurement of Mutarotation
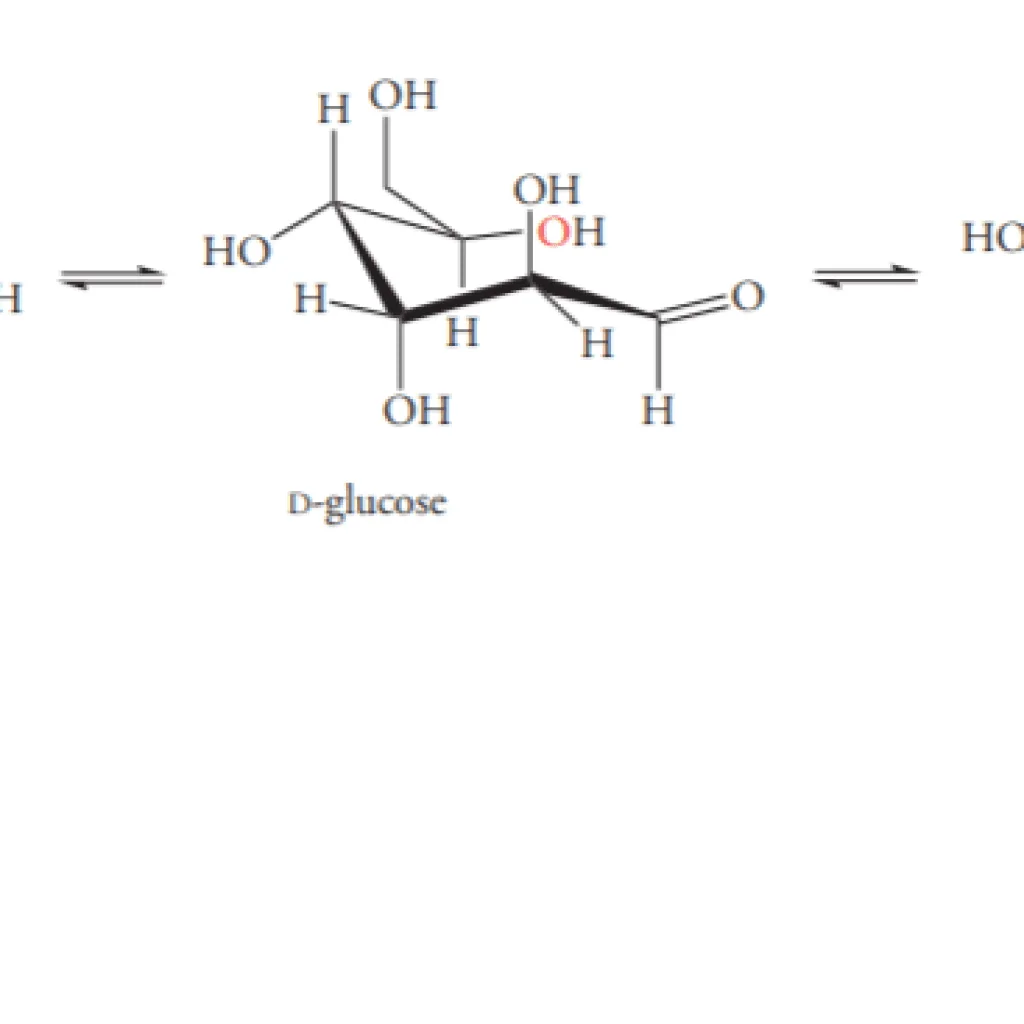
Mutarotation involves the mechanism of ring-chain tautomerism. The two different cyclic hemiacetal forms of sugars establish a state of equilibrium with the linear form. It means that even if a compound is 100% pure (containing only one form when it’s dissolved in water), it undergoes the equilibrium state with its linear pattern.
For example, when a 100% alpha-glucose form is added to water, it unmasks itself into a straight chain (or linear pattern).
And, when it reforms, it can either change into an alpha form or beta form. Further, after some time, an equilibrium state is achieved between both forms show that the reaction follows the zeroth law of thermodynamics.
The alpha and beta anomers of the sugars have different specific rotations. A liquid solution of the pure alpha compound will rotate at a different angle and in the opposite direction to that of the solution of the pure beta compound.
The individual value of the optical rotation of each anomer and their ratio in the solution determines the optical ratio of a solution.
The optical rotation of the sample is weighed by taking the sum of the optical rotation of each monomer. A polarimeter is used to measure the rotation of a sample. This can also be used to calculate the ratio of two forms of a compound (anomers) present in the solution.