

Risk-based monitoring (RBM) tools are essential in drug development and digital health research. Due to the complexity of medical research (with strict regulatory practices, documentation requirements, and ethical considerations), clinical trial monitoring is needed to ensure protocol compliance, data quality, and participant safety. To help sponsors and researchers overcome possible challenges and financial burdens, regulatory bodies worldwide agree that a risk-based approach in monitoring is crucial. The ICH-GCP guidelines, for instance, states that clinical trial observations and risk-based monitoring tools are mandatory in health research and drug development (Hurley et al., 2016).
Unlike standard burdensome procedures, such as on-site visits and source data verification, risk-based monitoring is highly effective. Risk-based monitoring is an innovative practice that can help researchers reduce the frequency of monitoring and incorporate different assessments in practice (e.g., on-site and remote monitoring). What’s more, it tackles various factors of research, such as high-risk sites, triggered events, data quality, and patient’s well-being.
Risk-based monitoring tools, in particular, allow experts to implement risk-based assessment, tailor their monitoring practices, reduce risks, and optimize clinical outcomes. Note that critical data and risks are often associated with data integrity, novel products, research bodies, study design, and participants’ safety. Risk-based monitoring tools can also support ongoing supervision in real time and improve long-term health benefits. They can help reshape the nature of pharmaceutical drug discovery and digital health.
Researchers worldwide agree that risk-based monitoring can improve drug development and patient outcomes. Risk-based monitoring tools can help researchers implement risk-based monitoring in practice and overcome challenges in software technology. They can improve the conduct, management, and audit of clinical trials at all phases of research. A recent literature review revealed that although there isn’t an existing set of regulations regarding risk-based practices, risk categories are normally defined as low, medium, and high-risk (Hurley et al., 2016). Note that low-risk studies require less data monitoring. Having clear categories and taxonomy can ensure trial accuracy, patients’ safety, and data collection. It’s interesting to mention that Phase I studies are usually flagged as a high-risk category.
The implementation of risk-based monitoring tools should also tackle different types of monitoring in research. Note that centralized monitoring, statistical assessment, reduced monitoring, remote observations, and on-site procedures are among the most effective monitoring techniques (Molloy & Henley, 2016). Interestingly, centralized monitoring is a major component of risk-based monitoring, which reveals numerous advantages over standard assessments.
In addition, Hurley and colleagues (2016) concluded that risk-based monitoring tools could be paper-based, operated as a Service as a System (SaaS) or supported by Excel. Note that with the recent shift in digital health practices, electronic data and digital solutions are becoming more and more popular in medical research. Most of all, experts should embrace the fact that risk assessment is a continuous process and requires real-time analysis to improve drug development and health outcomes.
For the successful implementation of risk-based monitoring tools, detection of critical data (which can be flagged as a risk) is crucial. The most common risks in medical research are linked to data integrity and ethical regulations. Interestingly, some experts suggest using a ‘traffic light system’ during risk assessments to identify and visualize low, medium, and high risks. One of the most popular taxonomies identifies 12 risks (Jongen et al., 2016), categorized into three groups:
What – Investigational medicinal products (IMP): When it comes to novel products, numerous risks can arise. One of the major unknowns in medical research revolves around the existing knowledge about new treatments in humans. Adverse effects and toxic compounds may be lethal. Since Phase I and Phase II trials pose high risks for participants, new indications and toxicological effects must be tested thoughtfully. Another major risk tackles the nature of the actual treatment. In trials where no medications for potential side effects are available, or where interventions continue without any benefits for participants, risks are high. Let’s not forget that patient safety and well-being come first. The third concern about an experimental product comes down to potential large populations. This is highly relevant for data integrity, and marketing authorization approval as a drug designed for big populations may harm a large number of people. The last challenge in the investigational medicinal products category tackles high-risk drugs. Note that there are three types of risk characteristics which a product may hold: pharmacology, route of administration, and stability. As stated above, new indications, possible side effects, and storage errors must be considered in order to reduce adverse effects and negative outcomes.
By whom – Investigators, site, sponsors: Research is a complex process which involves researchers, sponsors, participants, practitioners, and committees. When it comes to staff and sponsors, professionalism, experience, and reputation become crucial. In fact, these three characteristics may affect data integrity and ethical risks. Normally, commercial organizations and experienced researchers tend to identify risks and ensure data integrity. The reputation of an organization also affects risks and outcomes; it can affect approval decisions and market authorization. Thus, training of staff, good documentation practices, and protocols become vital.
How – Design: A safe drug and professional staff is not enough to guarantee success. The study design becomes crucial. Participants characteristics, for example, may correlate with risks. Trials with a large number of subjects (e.g., Phase III studies) pose both ethical concerns and risk for data integrity. When vulnerable people or participants across emergence settings are enrolled, risks also increase. Another aspect which is associated with high risk is the potentially high burden to participants. Invasive procedures and time-consuming methods, for example, often lead to high risks. The duration of the actual treatment also needs to be considered. Usually, longer treatments correlate with high occurrences of adverse effects. The actual study design is also a crucial factor. Studies with multiple phases, sites, and research bodies – as well as those that employ new assessment tools and complex procedures – pose a high risk for data integrity. Last but not least, the conduct of the study is essential. Any breaches of the protocol must be tackled to prevent possible failures and adverse effects. Good management and documentation practices become fundamental.
Risk-based monitoring tools are essential in clinical research. They reveal numerous benefits in practice, such as reduced costs, decreased time to approval of a new product, and lower risks. It’s not a secret that drug development is a complex process. It starts with research on a molecular level to support the understanding of the disease. Once a possible target and compounds have been identified, preclinical testing in non-humans can begin; followed by clinical trials in humans, marketing, and patenting (Torjesen, 2015). Unfortunately, stats show that approximately five in 5,000 drugs enter human testing; with only one of these five products being approved. The entire testing process is prone to delays (up to 12 years) and costly (more than $ 2.6 billion). Therefore, risk-based monitoring tools are needed to improve drug research:
High-quality data and sources: Risk-based monitoring tools can ensure high-quality data and benefit patients’ well-being. It’s not a secret that standards methods, such as source data verification, can be burdensome and ineffective. Due to the complexity of clinical research, risk-based approaches and centralized monitoring become essential. Novel assessments and tools can help experts focus on critical data and problematic sites in order to improve outcomes.
Reduced costs and delays: As explained above, pharmaceutical research is a costly business. Data shows that 1/3 of a study’s budget goes to on-site monitoring of data. Consequently, more and more regulatory bodies, including the FDA, implement risk-based monitoring tools to reduce costs, fraudulent data, and delays. Such risk-based monitoring tools can reduce the frequency of monitoring, decrease financial burdens, and improve data integrity.
Better outcomes and digital solutions: Risk-based monitoring tools can reduce the risks of errors. The use of automated reviews and a central risk dashboard can lead to better analysis and results. It also allows for cross-site comparison and interoperability. After all, digital solutions can improve health outcomes and reshape the entire nature of digital health research.
Although risk-based monitoring is fundamental in drug development, the lack of a consensus between regulatory bodies and research organizations may become problematic (Agrafiotis et al., 2018). For instance, the interchangeable use of terms, such as risk-based monitoring, centralized monitoring, and quality by design, is a clear example of the need for integrity and harmonization. Note that risk-based monitoring is defined “[RBM] directs sponsor oversight activities on preventing or mitigating important and likely risks to data quality and to processes critical to human subject protection and trial integrity by appropriate use of centralized monitoring and reliance on technological advances.” Therefore, it requires the implementation of both centralized monitoring and quality by design. To overcome research ambiguities and possible challenges, regulatory bodies state that any risk-based monitoring program should include:
Data collection: Any risk-based approach requires a robust flow of information (both automatic and manual), which can be transferred from each study site to their central monitoring system. Data collection in real time, 24/7, can provide essential medical information about patient outcomes, well-being, and financial burdens. Note that novel technologies, real-time data entry, and sophisticated monitors’ capabilities allow remote monitoring based on probability and simulations. This novel approach can help researchers and sponsors spot critical data or potential risks before the actual occurrence of an incident.
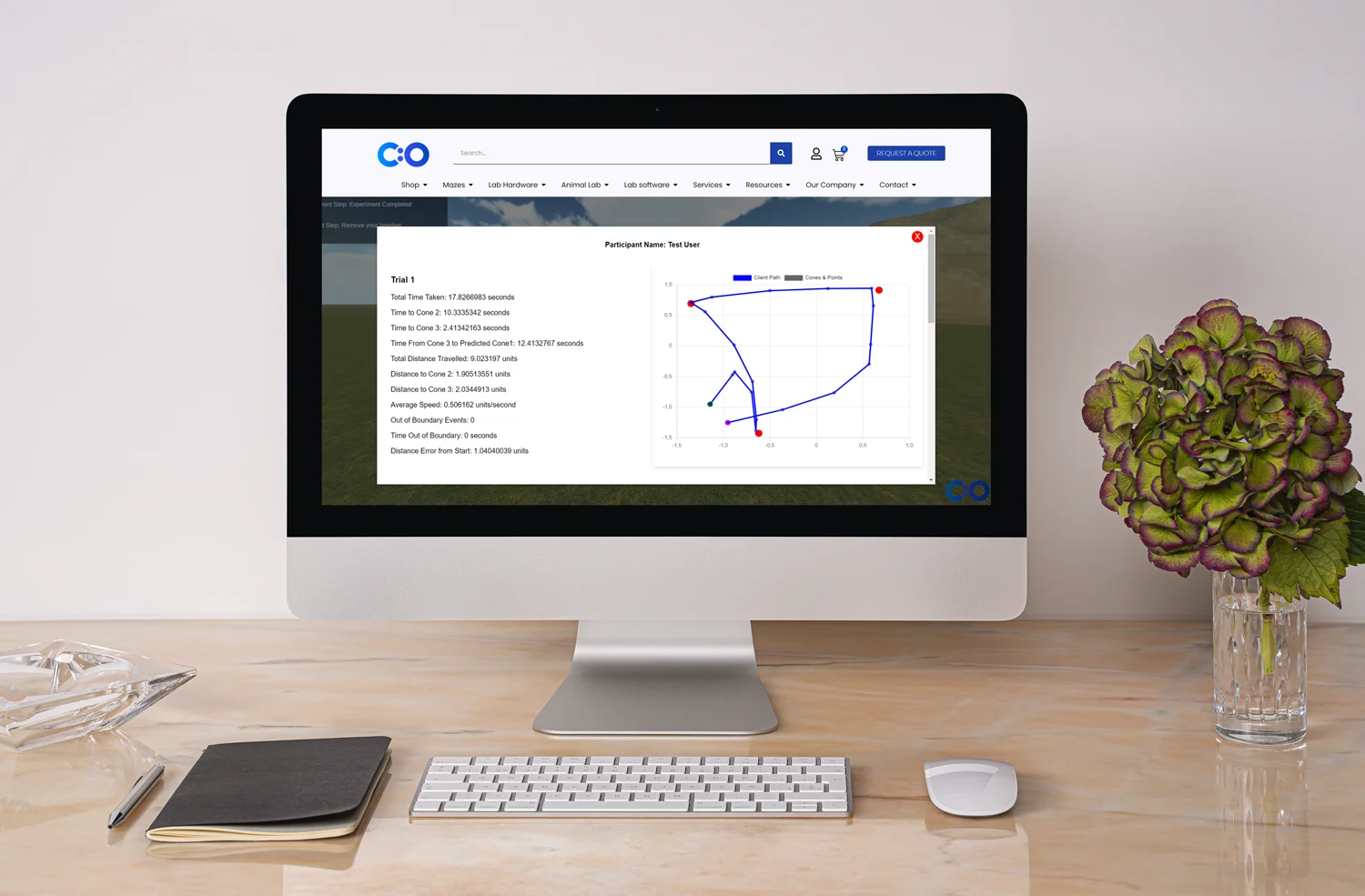
Dashboard monitoring: Experts agree that dashboard monitoring becomes an essential risk-based monitoring tool. It integrates medical data into a single platform, which allows automatic data flow and faster decision-making. A central dashboard also facilitates data collection and analysis. What’s more, dashboard monitoring can provide visuals about thresholds for given risks and solutions. Experts can customize (e.g., color-code) their system to categorize risks (e.g., by site). After all, visuals help researchers make sense of their rich data.
Statistical analysis and digital solutions: Risk-based monitoring tools help experts perform additional statistical analyses and create histograms and charts. Analyses depend on risk-based thinking and predictive analytics, which can reduce risks and triggers. Note that depending on the identified risks, a targeted on-site investigation and traditional source data verification may be required as an additional technique. As explained above, this can reduce costs, errors, and delays.
Since medical research and drug development are complex scientific endeavors, novel research methods are required. Risk-based monitoring, for instance, has numerous advantages over standard monitoring practices. Risk-based monitoring is defined as the process of ensuring data integrity and ethical principles. It moves away from traditional practices, such as source data verification, and employs sophisticated tools in order to assess clinical trials and novel interventions. New predictive analytics can help researchers identify risks and improve outcomes.
Risk-based monitoring tools help researchers implement risk-based monitoring, conduct an efficient clinical trial, reduce on-site visits, cut costs, and ensure data integrity. As the FDA defines three crucial steps in risk-based monitoring (identifying critical data, performing a risk assessment, and developing a monitoring plan), risk-based monitoring tools can simply support research teams. Note that technological advancements also benefit risk-based monitoring, centralized monitoring, predictive analytics, and visualization techniques. Innovative dashboards and online platforms such as Qolty can enhance data flow, risk-based monitoring, cross-site comparison, and research communication.
In the end, risk-based monitoring tools can reshape the nature of traditional clinical trials, improve patient safety, and ensure data quality.








Dr Louise Corscadden acts as Conduct Science’s Director of Science and Development and Academic Technology Transfer. Her background is in genetics, microbiology, neuroscience, and climate chemistry.
