
Safranin is a cationic dye used in histology and cytology to distinguish and identify different tissues and cells. It is popular in medical research for staining acidic proteoglycan that is found in cartilage tissues, enabling researchers to analyze cell chondrogenesis. The safranin is employed as a counter-stain in endospore staining and Gram’s staining. It is mostly utilized for the identification of cartilage, mucin, and mast cell granules.
The safranin stain works by binding to acidic proteoglycans in cartilage tissues with a high affinity forming a reddish orange complex. The binding made cartilage tissues appear red when observed under the microscope. The safranin staining helps the researchers detect not only cartilage tissues but also all the body tissues and organs. The safranin stain is one of the most promising safe-lab stains used for histopathological and cytological research.
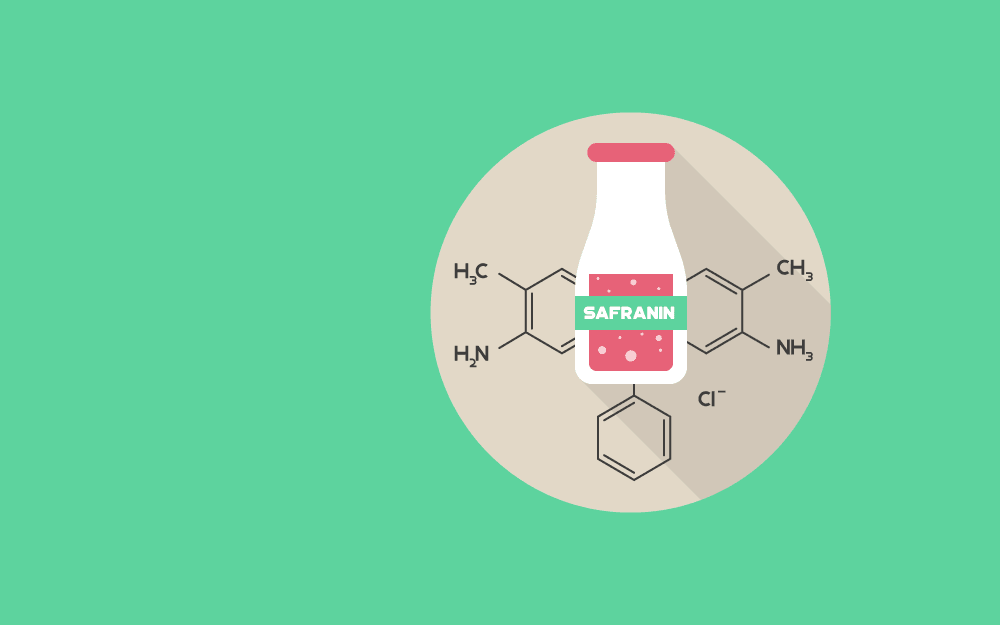
Safranin is a basic biological dye commonly used as a counter-stain in some of the staining protocols like gram staining. Following are the chemical and physical properties of safranin.
The following procedure should be followed for safranin staining.
To prepare the staining solution;
To prepare slides for observation;
The Gram’s staining is performed to distinguish between two large groups of bacteria depending on their cell wall constituents. These bacterial groups are named gram-positive and gram-negative bacteria. The presence of a thick peptidoglycan layer in the gram-positive bacteria causes it to appear purple while the thinner peptidoglycan layer in gram-negative bacteria causes it to appear pink after Gram’s staining. The safranin serves as a counterstain in gram staining. The procedure followed for gram’s staining is described below:
Johansen’s method is different from the safranin staining technique. The protocol is widely used for plant tissue analysis. When plant tissues are stained with Johansen’s method the chromosomes, nuclei, lignified, or cutinized cell walls appear bright red. The staining procedure is described below:
The safranin staining is the most widely used staining technique for cell differentiation, cell-based assays, and stem cell culture. The safranin stain is commonly used to quantify and identify the acidic proteoglycan and glycosaminoglycan in the cartilage tissues. The safranin staining helps the researchers detect not only cartilage tissues but also all the body tissues and organs. Following are some research applications of the safranin stain.
Safranin is a cheap and safe dye that has been predominantly used to stain plant tissues for histological analysis. It was then employed to diagnose frozen sections of basal and squamous cell carcinomas accurately. In the experiment, 1.0% lithium carbonate and a 0.5% safranin staining solution were used, which dyed the keratin a deep red, while staining the other structures like muscle cells, hair follicles, mast cell granules, and sebaceous glands pinkish red. As a result, the safranin staining provided the experimenters with high contrasting slides that are easier to analyze under the microscope for better diagnosis of frozen sections of basal and squamous cell carcinomas. The safranin staining is the most promising staining technique used for histological analysis and diagnosis of tumor cells with reasonable accuracy.
In another research, the safranin staining was used to examine the Cryostat microtome sections of birch wood degraded by white-rot fungi. In the experiment, safranin was used in combination with Astra-blue. The safranin stained the lignin irrespective of the presence of cellulose, while the Astra-blue dyed the cellulose blue only in the absence of lignin. Then, the slides were observed under light microscopy. The method provided the researchers with a comfortable and reliable screening procedure to distinguish between fungi degrading cellulose and lignin, and fungi degrading cellulose only. The technique offered the understudies a detailed morphological and histological analysis of the plant cells.
The safranin staining has helped the researchers to develop a new micro-spectrophotometric method for quantitation of glycosaminoglycan in cartilage tissues. The dye content of the tissues was proportional to the amount of glycosaminoglycan in the cartilage matrix. With the help of gas chromatography and thin-layer chromatography, the fixed negative charge contained in the analyte was determined. Safranin staining is the most commonly used method to detect and analyze cell chondrogenesis.
The safranin is also used as a counter-stain in Gram’s staining. In Gram’s staining, the safranin directly stains the bacteria that has been decolorized. With safranin staining, gram-negative bacteria can be easily distinguished from gram-positive bacteria.
The above-mentioned research applications of the safranin stain show that the scope of safranin staining is not only limited to plant histology, but also microbiology, oncology, stem cell differentiation, and arthrology.
