
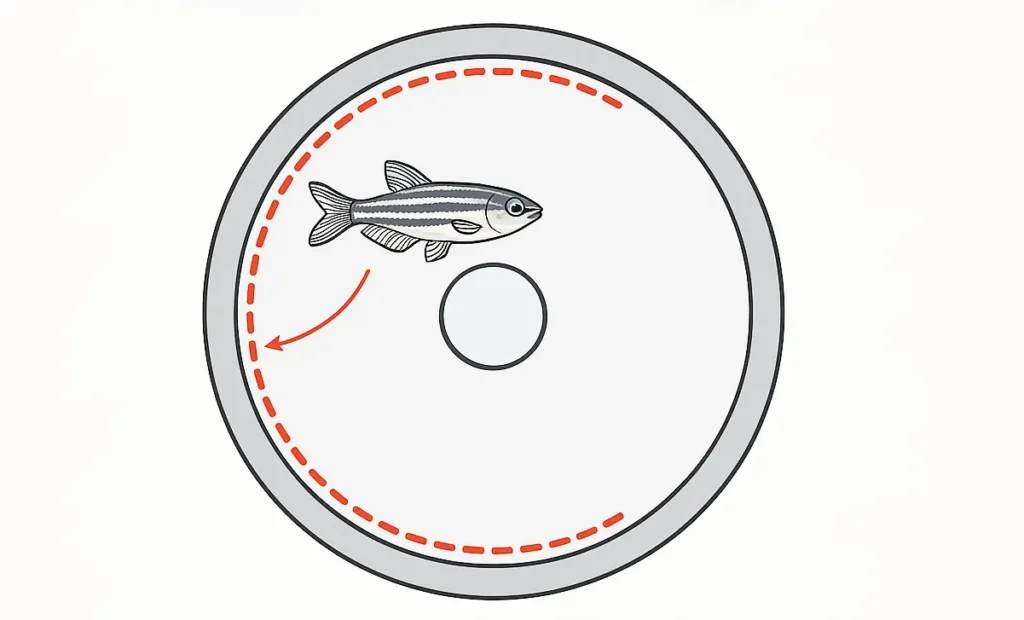
In behavioral neuroscience, the path a subject avoids is often as telling as the path it chooses. Within the context of zebrafish research using the Visual Water Maze, one such behavior—thigmotaxis, or wall-hugging—is especially revealing. Although sometimes dismissed as an artifact or a peripheral behavioral quirk, thigmotaxis holds critical value as an emotional and cognitive barometer. It tells us not only about a zebrafish’s stress levels but also how those levels may interfere with learning, memory, and spatial decision-making.
Defined as the tendency to remain close to the tank walls, thigmotaxis is measured as the time spent in the periphery of the arena, usually within 1–2 cm of the outer boundary. In zebrafish, elevated thigmotaxis is widely recognized as a behavioral correlate of anxiety, particularly in novel environments or under stress-inducing conditions. But more than just a marker of fear, it is also a behavioral confound—one that can compromise task performance and obscure true cognitive capability if not properly understood and controlled.
Thigmotaxis originates from the Greek word thigma, meaning “touch.” It refers to the instinctive tendency of animals to stay close to vertical surfaces—a behavior observed in species from rodents to fish. In zebrafish, thigmotaxis is expressed as swimming along the edges of the tank, particularly during initial exposure to a novel or stressful environment. It is often accompanied by reduced exploration of the center area, decreased platform-seeking behavior, and in some cases, freezing or erratic swimming.
In the Visual Water Maze, thigmotaxis becomes apparent when a fish spends a disproportionate amount of time in the wall-adjacent zone, even after multiple training sessions. While brief periods of thigmotaxis are normal during the habituation phase, persistent or excessive thigmotaxis may indicate elevated anxiety or impaired environmental adaptation, both of which can interfere with spatial learning.
The primary concern with thigmotaxis in spatial learning assays is that it competes with task-relevant behavior. A zebrafish that hugs the wall is:
This means that high thigmotaxis can falsely mimic cognitive impairment, even when memory function is intact. If a fish knows where the platform is but is too anxious to leave the wall, the resulting behavior may be misinterpreted as a learning deficit.
Studies have shown that environmental stressors, including sudden lighting changes, vibrations, or unfamiliar handling, can increase thigmotaxis and reduce maze performance, underscoring the importance of standardized experimental protocols and environmental habituation.
While often viewed as a nuisance variable, thigmotaxis is also a valuable endpoint in its own right, especially in studies targeting:
In such cases, a decrease in thigmotaxis over time can serve as a behavioral readout of habituation and emotional adaptation, while persistent thigmotaxis may reflect trait-level anxiety or chronic stress phenotypes.
In videos from the Conduct Science YouTube channel, zebrafish exhibiting strong wall-hugging behavior are often observed during early maze exposure or in pharmacological tests involving anxiogenic compounds—highlighting the utility of this behavior as a real-time emotional indicator.
Measuring thigmotaxis, or wall-hugging behavior, is critical in zebrafish cognitive and affective research. Within the Visual Water Maze, thigmotaxis is not only a behavioral phenotype but also a variable that can profoundly influence performance on tasks designed to assess learning and memory. As such, its measurement must be carried out with precision, standardization, and contextual awareness.
Conduct Science’s Visual Water Maze system is optimized to track thigmotaxis with high accuracy, offering real-time data collection and customizable zone definition. This allows researchers to isolate and analyze thigmotaxis as an independent behavioral metric, providing critical insight into the animal’s anxiety levels, environmental adaptation, and engagement with the task.
The first step in measuring thigmotaxis is to define the peripheral zone of the maze, commonly referred to as the thigmotaxis zone. This area represents the wall-adjacent region of the tank and is where anxiety-like behavior typically manifests.
Best practices for zone definition:
The defined thigmotaxis zone becomes the spatial anchor for detecting when a fish is exhibiting wall-hugging behavior versus exploratory or goal-directed movement.
Conduct Science’s integrated tracking system captures zebrafish movement via high-resolution video and real-time x-y coordinate mapping. As the fish swims, the software continuously monitors its position relative to the tank zones.
Thigmotaxis is typically quantified as:
Researchers can also segment thigmotaxis data by:
This allows for tracking emotional adaptation, habituation, or drug-induced changes in anxiety-like behavior.
The system provides comprehensive numerical and visual outputs, which include:
These tools support not only descriptive analysis but also hypothesis-driven comparisons between experimental groups, time points, or treatment conditions.
To fully interpret the role of thigmotaxis in the Visual Water Maze, it should be analyzed alongside cognitive performance measures such as:
For instance:
This integrative approach provides a composite view of the zebrafish’s cognitive-emotional profile, allowing researchers to draw more accurate and nuanced conclusions.
To ensure thigmotaxis measurement is both valid and reproducible, adhere to the following best practices:
When properly measured, thigmotaxis is not a nuisance variable, but rather a behavioral signal—one that reveals how zebrafish perceive, adapt to, and interact with their testing environment.
In the Visual Water Maze, wall-hugging behavior is more than a side note—it’s a window into the animal’s emotional state, and a factor that can directly affect how spatial memory is expressed. By leveraging Conduct Science’s advanced tracking and analysis tools, researchers can quantify thigmotaxis with high spatial and temporal resolution, ensuring that this behavior is accurately accounted for and thoughtfully interpreted.
With precise measurement and contextual integration, thigmotaxis becomes a core component of zebrafish neurobehavioral assessment, offering insight into both the mind and the mood behind the maze.
In cognitive research using the Visual Water Maze, thigmotaxis must be interpreted with nuance, as it can profoundly affect the behavioral readouts typically used to assess learning and memory. When a zebrafish displays high thigmotaxis—spending a substantial portion of the trial near the tank walls—it may not be due to a failure in memory or spatial learning per se. Rather, it could reflect anxiety, poor environmental habituation, or an altered affective state, which causes the animal to avoid open areas of the maze where the platform is located. This behavior, while adaptive in natural contexts, becomes a cognitive confound in spatial tasks where central zone exploration is essential for success.
Persistent thigmotaxis can artificially inflate escape latency, reduce platform crossings, and bias path metrics, leading to the misclassification of an emotionally stressed animal as cognitively impaired. It may also disrupt the animal’s ability to effectively use visual cues, especially if the cues are placed toward the center or are spatially associated with the hidden platform. In this context, high thigmotaxis suggests that the animal is not fully engaging with the task or is avoiding spatial decision-making opportunities, not necessarily failing to learn.
Conversely, low or diminishing thigmotaxis across trials is often an indicator of successful habituation, emotional regulation, and cognitive task engagement. Zebrafish that display reduced wall-hugging over time are typically better adapted to the maze environment, more likely to explore the full arena, and more responsive to visual cues. These animals show clear learning curves, often reflected in decreased escape latency, improved path efficiency, and more frequent platform crossings.
Importantly, thigmotaxis should always be considered in conjunction with other behavioral variables. For example, a fish with low thigmotaxis but high latency may truly have impaired spatial memory. Meanwhile, a fish with high thigmotaxis and poor performance might simply be overwhelmed or anxious, not incapable of learning. Swim speed, trajectory type, and quadrant preference can help contextualize these patterns and prevent misinterpretation.
In sum, thigmotaxis is both a behavioral variable and a cognitive modulator. High wall-hugging may signal affective interference, while low thigmotaxis reflects cognitive readiness. Interpreting this behavior correctly ensures that assessments of zebrafish memory are not clouded by emotional artifacts and that task performance is evaluated within a complete behavioral and psychological framework.
Thigmotaxis—wall-hugging behavior—offers a powerful lens through which researchers can evaluate both emotional reactivity and cognitive performance in zebrafish. Within the Visual Water Maze, this behavior functions not only as a potential confound in learning tasks, but also as an informative neurobehavioral endpoint in its own right. As zebrafish models continue to expand across fields such as neuropsychiatry, neuropharmacology, toxicology, and aging, thigmotaxis provides a non-invasive, real-time measure of internal states like anxiety, stress resilience, and environmental adaptability.
Thigmotaxis is one of the most consistent and robust behavioral markers of anxiety-like states in zebrafish. In the Visual Water Maze, elevated thigmotaxis is often observed during early trials or following exposure to stress-inducing stimuli, such as bright lighting, abrupt water changes, or novel environments.
This behavior becomes particularly valuable in studies of:
For example, zebrafish exposed to known anxiogenic compounds (e.g., caffeine, pentylenetetrazol) frequently demonstrate sustained thigmotaxis, even when learning requirements are minimal. Conversely, treatment with anxiolytics like fluoxetine or diazepam typically results in reduced wall-hugging, often before cognitive measures like platform crossings or escape latency begin to change.
Thus, thigmotaxis serves as an early emotional marker, allowing researchers to assess how internal states modulate spatial task engagement.
One of thigmotaxis’s most important contributions is its ability to highlight the interaction between emotion and cognition. A zebrafish may possess the spatial memory required to complete a task but may fail to express it due to heightened anxiety or reduced motivation—reflected in elevated thigmotaxis.
This makes the behavior particularly useful in:
Researchers can analyze thigmotaxis alongside metrics like path efficiency and platform crossings to discern whether performance deficits stem from true memory impairment or emotional interference. This level of interpretive depth enhances the accuracy of conclusions drawn from complex behavioral datasets.
In pharmacological testing, thigmotaxis is increasingly used to evaluate the side effect profile and behavioral impact of new compounds. Because it is sensitive to both neurochemical modulation and task-related stress, thigmotaxis can serve as a screening tool for identifying treatments that either alleviate or exacerbate emotional distress.
Applications include:
A reduction in thigmotaxis in treated groups often correlates with enhanced cognitive performance, while persistent thigmotaxis can reveal a hidden cost of pharmacological intervention that may not be immediately apparent in spatial accuracy metrics.
Thigmotaxis is also gaining recognition in toxicological and environmental exposure research. Zebrafish exposed to environmental toxins—such as heavy metals, endocrine disruptors (e.g., BPA), or neurotoxic pesticides—often display elevated thigmotaxis even in the absence of motor deficits.
This behavior may reflect:
By measuring thigmotaxis alongside other Visual Water Maze metrics, researchers can detect sublethal effects of environmental agents that specifically impact emotional regulation and task engagement, making it a key component in ecotoxicological risk assessment.
In longitudinal studies, thigmotaxis provides a dynamic readout of environmental adaptation. Younger, healthy zebrafish typically exhibit high thigmotaxis on initial exposure, which decreases as they habituate to the maze environment. However, aged or cognitively impaired fish often show prolonged or persistent thigmotaxis, suggesting either delayed adaptation or chronic stress sensitivity.
This makes wall-hugging behavior especially useful in:
Tracking changes in thigmotaxis over time can reveal patterns of neural plasticity, learning adaptation, or emotional rigidity, offering insights into how aging and experience shape the brain’s response to challenge.
Far from being a mere nuisance variable, thigmotaxis is a powerful behavioral biomarker with wide-reaching applications in neurobehavioral research. Whether reflecting anxiety in response to a novel maze, emotional side effects of a drug, or stress-induced learning interference, this behavior reveals much about how zebrafish feel and how they learn.
By measuring and interpreting thigmotaxis with scientific rigor, researchers can enrich their understanding of emotion-cognition interactions, improve the precision of behavioral assays, and expand the translational relevance of zebrafish studies in mental health, toxicology, and aging.
Ensuring Valid, Reproducible, and Insightful Thigmotaxis Data
Thigmotaxis, as a behavioral marker of anxiety and environmental adaptation, must be analyzed and reported with careful methodological detail. When included in cognitive research using the Visual Water Maze, thigmotaxis not only contextualizes learning outcomes but also helps distinguish emotional interference from true memory impairments. To ensure that findings are scientifically meaningful and reproducible across studies, the following best practices should be followed for both analysis and reporting.
Establish and describe the spatial parameters used to define thigmotaxis in the arena. This typically involves a boundary zone adjacent to the tank wall, such as 1–2 cm from the edge.
Best practice:
This ensures that other researchers can replicate the spatial conditions and interpret data with full transparency.
Environmental variables—including lighting intensity, water depth, arena size, and background contrast—can significantly influence thigmotaxis behavior.
Recommendations:
Document these conditions explicitly in the methods to allow reproducibility and to support the interpretation of anxiety-related behavior.
Always report both raw and normalized thigmotaxis data. Include:
This combination of absolute and relative measures allows for a more comprehensive interpretation, distinguishing between persistent occupation and repeated brief visits.
Thigmotaxis should not be analyzed in isolation. Integrate it with other behavioral indicators to build a multi-dimensional behavioral profile.
Essential pairings:
These pairings help identify whether thigmotaxis is a primary emotional feature or a secondary consequence of impaired cognition.
Numerical thigmotaxis data should be supported by visual representations for clearer interpretation and communication.
Recommended visual tools:
These visuals help validate automated scoring and support more intuitive comparisons between treatment groups or time points.
Where possible, assess thigmotaxis across multiple trials or days to monitor:
Reporting changes in thigmotaxis over time enhances the understanding of emotional flexibility and learning capacity.
Since thigmotaxis data can be non-normally distributed, especially with high inter-individual variability, statistical analysis should be selected accordingly.
Recommendations:
Clear statistical reporting strengthens the credibility of your findings and supports meta-analytic comparisons.
To ensure full transparency:
This level of detail promotes open science practices and enables other labs to replicate your analysis pipeline accurately.
Analyzing and reporting thigmotaxis in the Visual Water Maze is not just a technical requirement—it’s a scientific opportunity. When measured with precision and interpreted with context, thigmotaxis provides deep insight into the emotional states that shape zebrafish behavior and influence cognitive performance.
By adhering to these best practices, researchers ensure their results are not only valid but also transparent, interpretable, and impactful, contributing to a richer understanding of cognition-emotion interactions in the zebrafish model.
Thigmotaxis in zebrafish is more than a peripheral movement pattern—it is an emotional signal and a cognitive modulator. In the Visual Water Maze, time spent near the walls can reveal as much about anxiety, stress adaptation, and memory interference as direct performance metrics do.
By carefully measuring and interpreting thigmotaxis, researchers can disentangle cognitive from emotional behavior, ensuring their findings reflect true learning rather than stress-induced artifacts. For those probing the intersection of memory, emotion, and behavior in zebrafish, thigmotaxis offers an essential window into the brain’s internal state—seen clearly at the outer edge of the tank.
Blaser, R. E., Chadwick, L., & McGinnis, G. C. (2010). Behavioral measures of anxiety in zebrafish (Danio rerio). Behavioural Brain Research, 208(1), 56–62. https://doi.org/10.1016/j.bbr.2009.11.009
Colwill, R. M., & Creton, R. (2011). Locomotor behaviors in zebrafish (Danio rerio) larvae. Behavioural Processes, 86(2), 222–229. https://doi.org/10.1016/j.beproc.2010.12.003
Eddins, D., Cerutti, D., Williams, P., Linney, E., & Levin, E. D. (2010). Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure. Neurotoxicology and Teratology, 32(1), 99–105. https://doi.org/10.1016/j.ntt.2009.04.070
Kysil, E. V., Meshalkina, D. A., Frick, E. E., Echevarria, D. J., Rosemberg, D. B., Maximino, C., … & Kalueff, A. V. (2017). Comparative analysis of zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish, 14(3), 197–208. https://doi.org/10.1089/zeb.2016.1415
Maximino, C., da Silva, A. W., Gouveia, A., & Herculano, A. M. (2011). Pharmacological analysis of zebrafish (Danio rerio) scototaxis. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 35(2), 624–631. https://doi.org/10.1016/j.pnpbp.2011.01.006
Parker, M. O., Millington, M. E., Combe, F. J., & Brennan, C. H. (2012). Development and automation of a test of impulse control in adult zebrafish. Journal of Neuroscience Methods, 210(2), 196–205. https://doi.org/10.1016/j.jneumeth.2012.07.019
Stewart, A. M., Wong, K., Cachat, J., Gaikwad, S., & Kalueff, A. V. (2011). Developing zebrafish models relevant to PTSD and other stress-related disorders. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 35(6), 1421–1431. https://doi.org/10.1016/j.pnpbp.2010.09.017
Tang, W., Wu, J., Ying, Y., Jiang, Y., & Yang, H. (2020). Modeling autism spectrum disorder in zebrafish: A behavioral and neuropharmacological perspective. Neuropharmacology, 171, 108082. https://doi.org/10.1016/j.neuropharm.2020.108082

Monday – Friday
9 AM – 5 PM EST
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.