
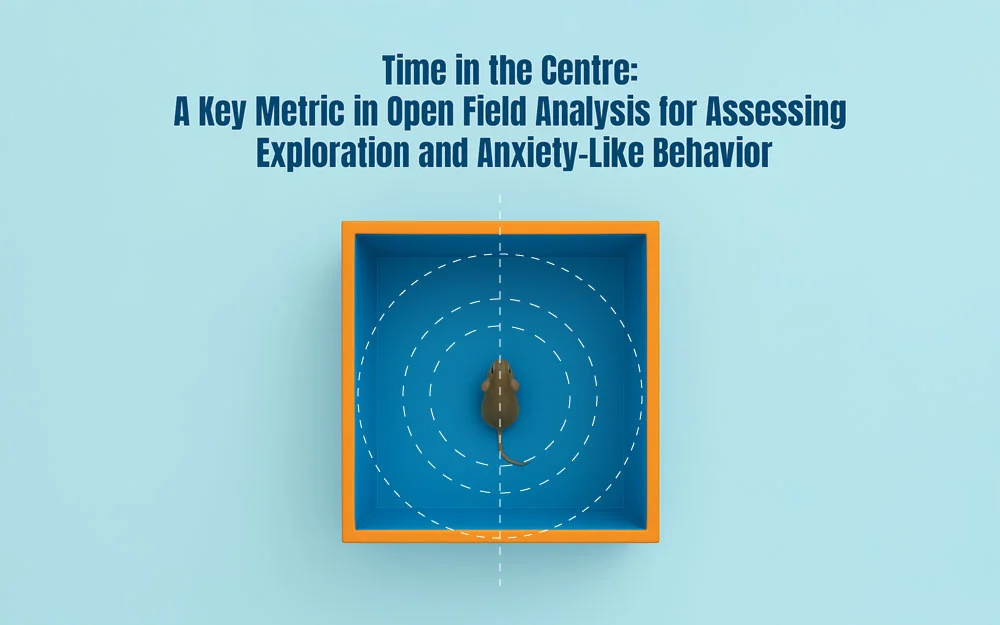
The Open Field Test (OFT) is a foundational behavioral assay used in neuroscience and psychology to evaluate general locomotor activity, anxiety-like responses, and exploratory tendencies in rodents. Among the most informative and interpretable metrics in this paradigm is Time in the Centre, which measures the duration an animal spends in the central region of the open field arena.
This spatially defined metric offers a powerful, non-invasive readout of emotional and cognitive states. Animals typically perceive open, exposed spaces as riskier due to predation threats, making the center zone a psychologically aversive area. As such, the amount of time spent in the center serves as a reliable indicator of anxiety-like behavior, risk assessment, and exploratory motivation.
Time in the centre quantifies how long an animal remains within the central portion of the open field arena, typically defined as the inner 25–50% of the total area depending on the arena’s size and shape. It captures a key behavioral dichotomy: approach versus avoidance. In rodents, the centre zone represents a space of elevated psychological vulnerability—far from the safety of the walls, more exposed, and thus more anxiogenic.
This measure reflects several interconnected behavioral dimensions:
Time in the centre thus functions as a multi-dimensional behavioral index, shaped by the interaction of anxiety, arousal, cognition, and sensorimotor processes. Its power lies in its simplicity and its sensitivity to a broad spectrum of psychological and neurological variables. When paired with complementary measures, it enables researchers to dissect the emotional and motivational architecture of exploratory behavior in rodents.. It reflects a behavioral trade-off between risk aversion and exploratory drive:
This behavior is driven by the rodent’s natural preference for enclosed, protected spaces (thigmotaxis), where proximity to walls is associated with safety. Thus, venturing into and remaining in the center suggests a behavioral shift toward approach over avoidance.
The measurement of time in the centre during Open Field Testing offers a robust behavioral lens into the internal emotional and cognitive states of animals. It is one of the most well-established metrics for assessing both state and trait anxiety, but its relevance extends far beyond this single domain. Time in the centre functions as a high-throughput, non-invasive index of emotional valence, exploratory decision-making, and neural circuitry function. Its broad applicability makes it a cornerstone metric in both mechanistic and translational studies, informing everything from pharmacological screening to gene-by-environment interactions.
Time in the centre is one of the most validated measures of unconditioned anxiety-like behavior in rodents. The metric is sensitive to both acute and chronic manipulations that alter emotional reactivity. For example, acute exposure to predator scent or restraint stress typically leads to a rapid reduction in centre time, reflecting enhanced avoidance behavior. Conversely, chronic administration of anxiolytic agents such as benzodiazepines, SSRIs, or GABAergic compounds significantly increases centre occupancy. These bidirectional effects make it a gold standard for quantifying emotional responsiveness and evaluating treatment efficacy in preclinical models of anxiety, PTSD, and stress reactivity.
The most widespread application of time-in-centre analysis is in preclinical models of anxiety and stress. Anxiogenic manipulations (e.g., predator odor, restraint stress, corticotropin-releasing hormone agonists) consistently reduce time in the centre, while anxiolytic treatments (e.g., benzodiazepines, SSRIs, GABA modulators) increase it (Prut & Belzung, 2003).
Genetically modified rodents, particularly those with mutations affecting the serotonergic, dopaminergic, or GABAergic systems, often exhibit altered center-related behaviors. Time in the centre helps identify behavioral phenotypes that correspond with specific neurochemical or molecular alterations. For instance, mice lacking 5-HT1A receptors show exaggerated center avoidance, consistent with hyper-anxious phenotypes. This metric is also useful for characterizing polygenic models of psychiatric disease and for parsing gene-environment interactions by observing how genetic risk factors manifest under different environmental conditions.
Knockout or transgenic animals with mutations in genes regulating neurotransmitter systems (e.g., serotonin, dopamine, GABA) often exhibit altered center behavior. For instance, 5-HT1A receptor knockouts tend to show center avoidance, reflecting enhanced anxiety phenotypes (Gross et al., 2002).
Time in the centre also reflects an animal’s willingness to engage with novel and potentially risky environments. High centre occupancy, especially in the absence of anxiolytic treatment, can signal high intrinsic novelty-seeking behavior or elevated exploratory motivation. This makes the metric particularly useful in addiction research and reward sensitivity studies, where animals with high exploratory drive may demonstrate altered drug-seeking behaviors. It also has relevance for impulsivity research, as centre preference may reflect lowered threat sensitivity or deficient inhibitory control.
In addition to anxiety, time in the centre is informative of motivational states. Animals with high novelty-seeking behavior may spend more time in the center due to increased drive to explore or reduced threat sensitivity. This makes the metric relevant in studies of impulsivity, addiction vulnerability, and reward processing.
Time in the centre also provides a non-invasive window into age-related changes in emotional regulation, exploration, and sensorimotor processing. In developmental studies, juvenile rodents often show increased centre engagement, reflecting higher novelty-seeking tendencies. Conversely, aged animals may exhibit reduced centre time due to anxiety, diminished motor function, or cognitive inflexibility. Longitudinal studies utilizing this metric can track developmental trajectories or age-related decline in affective and exploratory behavior over time.
Environmental and social factors can profoundly affect anxiety-like behavior. Social isolation, maternal separation, or enriched environments significantly alter time in the centre, with isolated animals typically displaying increased avoidance and enriched animals showing more centre entries and longer dwell times. These findings underscore the utility of this metric in research on neuroplasticity, resilience, and environmental interventions.
Time in the centre is frequently used to evaluate the role of specific brain regions—such as the amygdala, prefrontal cortex, and hippocampus—in regulating affective behavior. Techniques such as optogenetics, chemogenetics, and region-specific pharmacological inactivation use this metric to verify the behavioral outcome of targeted neural manipulations.
There is increasing recognition that sex hormones modulate anxiety-like behavior. Female rodents, depending on the estrous cycle phase, often exhibit different patterns of centre exploration compared to males. The metric is valuable in parsing out hormonal effects in studies of estrogen, progesterone, and testosterone on affective behavior.
Age-related changes in exploratory behavior are often reflected in reduced center entries and time. Juvenile animals generally spend more time in the centre than older counterparts, aligning with differences in risk-taking and sensorimotor function.
To ensure that time in the centre reflects meaningful behavioral differences, standardization and precise measurement are essential:
While time in the centre is widely associated with anxiety-like behavior, it represents a more nuanced and multifactorial behavioral outcome. To avoid misinterpretation, researchers must consider this measure within a broader behavioral context and understand the various underlying factors that can shape center-related behavior. Time in the centre is best viewed not as a single-output measure of anxiety, but as a reflection of dynamic interactions between locomotor activity, emotional state, memory, and environmental familiarity.
Several non-anxiety-related factors can influence center behavior:
To properly interpret time in the centre, it should be paired with complementary behavioral indices:
In sum, interpreting time in the centre requires a holistic approach. When contextualized with other data and methodological controls, this measure becomes a powerful window into the interaction of emotion, cognition, and behavior in animal models.. Several factors can influence center-related behavior:
To distinguish anxiety-specific effects from confounding variables, time in the centre should be analyzed alongside the following complementary metrics:
Together, these measures form a robust behavioral matrix that enables researchers to parse out whether changes in centre time are due to genuine anxiety-like behavior or other influencing factors such as locomotor activity, decision-making strategy, or sensorimotor capacity.
Time in the centre has become a widely adopted measure in translational models of mental health, offering a non-invasive and reliable behavioral readout in both early discovery and therapeutic validation stages. Below are key areas where this metric holds critical relevance:
These applications position time in the centre not only as a robust behavioral measure of affective state, but also as a translationally relevant metric with growing importance in therapeutic development, systems neuroscience, and environmental health research. Time in the centre is commonly used to screen anxiolytic or anxiogenic effects of new compounds.
Therapeutic Monitoring: This metric helps assess how interventions (e.g., exercise, optogenetics, deep brain stimulation) alter anxiety levels over time.
Time in the centre complements a suite of related measures to build a complete behavioral profile:
This multi-dimensional approach improves interpretation and reproducibility in both mechanistic and pharmacological studies.
Modern open field platforms are equipped with automated tracking systems capable of detecting subtle changes in zone dwell times and movement dynamics. Integrating these tools with controlled environments and standardized testing protocols enhances the quality and reproducibility of center-related metrics.
To explore validated open field testing systems that optimize anxiety and exploration research, visit the Open Field Test page and discover tools built for high-resolution behavioral insight.
Written by researchers, for researchers — powered by Conduct Science.










Dr Louise Corscadden acts as Conduct Science’s Director of Science and Development and Academic Technology Transfer. Her background is in genetics, microbiology, neuroscience, and climate chemistry.
