
Neuroscience Research:
Pharmacological Research:
Behavioral Studies:
Advanced Neuroscience Techniques:
Clinical Applications:
Developmental Studies:
Embryonic and Neonatal Research: The apparatus can be adapted for use with very small animal subjects, such as embryos or neonates, facilitating studies on brain development and the effects of early-life interventions [14].
The stereotaxic apparatus is frequently utilized in Parkinson’s disease research, as demonstrated in a recent study by Landeck et al. (2021).
Parkinson’s disease (PD) is a progressive disorder traditionally defined by resting tremor and akinesia, primarily due to the loss of dopaminergic neurons in the substantia nigra. Studies using stereotaxic injections deliver chemicals, proteins, or viral vectors to mimic various aspects of PD [15].
By injecting 6-OHDA (oxidopamine) into the brain, researchers caused a major loss of dopaminergic neurons, resulting in significant motor deficits. 6-OHDA is taken up by monoamine transporters and blocks mitochondrial respiration [15].
By destroying nigral dopaminergic neurons, the primary symptoms of Parkinson’s disease can be replicated, making rats used in stereotaxic surgery an effective model for Parkinson’s disease research.
Stereotaxic surgery is also utilized to create animal models of depression, which are essential for studying the underlying mechanisms and testing potential treatments for the disorder. Here’s how it can be done:
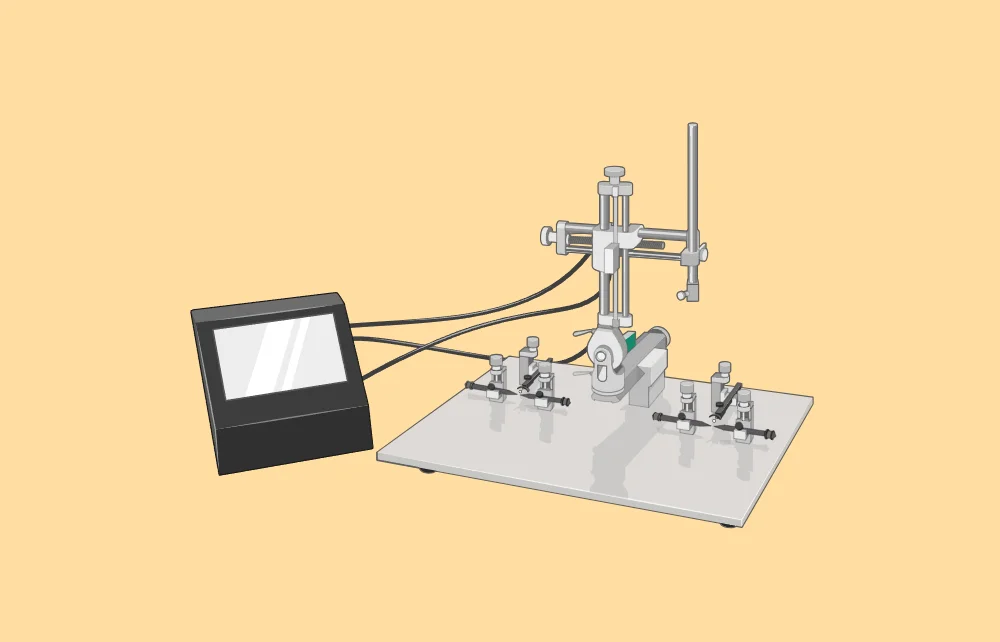
The stereotaxic apparatus is a key tool in modern neuroscience and biomedical research, allowing for precise targeting of specific brain regions. It is used in a variety of applications, including basic neuroscience research where it helps map the brain and study neurotransmitter systems, as well as in behavioral studies that create controlled lesions or involve the long-term implantation of devices for observation. In pharmacological research, the apparatus enables the accurate delivery of drugs and gene therapy vectors to specific brain areas, which is crucial for understanding their effects on neural circuits. It is also essential for advanced techniques like optogenetics, fiber photometry, and two-photon imaging, ensuring the correct placement of equipment needed for these methods. Clinically, the stereotaxic apparatus is used in procedures such as deep brain stimulation for Parkinson’s disease, where precise electrode placement is necessary for effective treatment. Additionally, it can be adapted for use in developmental studies with embryos or neonates, providing insights into brain development and early-life interventions. Overall, the stereotaxic apparatus is a vital tool that advances our understanding of the brain and aids in developing new treatments for neurological disorders.




Dr Louise Corscadden acts as Conduct Science’s Director of Science and Development and Academic Technology Transfer. Her background is in genetics, microbiology, neuroscience, and climate chemistry.
