
What are acids and bases? These are the chemicals that make water either acidic or alkaline. Acids make water acidic, while bases make it alkaline.
To understand acids and bases properly though, we’ll need to look at water first. Water is a simple, yet complicated chemical. We’ve all heard that the formula for water is H2O. But a solution of pure H2O doesn’t stay as just H2O. A very small amount of the water molecules spontaneously split into equal parts of H+ and OH–.
The H+ is a hydrogen ion, which is a hydrogen atom that has lost its electron, leaving only the proton. And the remaining OH– is called a hydroxide ion. Water with equal parts H+ and OH– is considered neutral.
With that understood, we can now clearly define acids and bases.
Acids are chemicals that, when dissolved in water, increase the amount of H+ ions, while bases increase the OH– ions. So, water containing more H+ is more acidic, and when it contains more OH–, the water is more alkaline.
Let’s take a closer look at both.
Acids are more familiar to most people, so let’s start there. Acids are chemicals that increase the amount or concentration of H+.
There are, in fact, three models the describe how acids increase H+. The simplest is the Arrhenius model, which defines an acid as a chemical that “releases” H+ into water. Similarly, the Brønsted-Lowry model defines an acid as a chemical that “donates” H+.
Finally, the Lewis model defines an acid as a chemical that can accept negatively charged electrons. H+ is a Lewis acid because it can accept electrons from water (H2O) forming a hydronium ion or H3O+. While this definition is the most comprehensive, it’s also technically challenging, so it’s easier to think of acids releasing or donating H+.
The acids that release all of their H+ are called strong acids, while others that release only a tiny fraction, are known as weak acids.
Strong acids release all their H+ into water. The most familiar strong acids are hydrochloric acid (HCl) and sulfuric acid (H2SO4). In water, HCl splits entirely into H+ and Cl–, or chloride (think of chloride from table salt). It’s strong because all the HCl added will become H+.
Strong acids are dangerous because they increase the concentration of H+ so much that it chemically reacts with many things, including our bodies. Strong acids can be very corrosive, so extra precaution is needed when using them.
The most well-known strong acid is hydrochloric acid (HCl). It’s the acid in our stomachs required for digestion and protection from microbial infection. It’s also used in high-strength cleaning products from toilet bowl cleansers to rust removal in steel production.
Likewise, sulfuric acid (H2SO4) is used in strong drain cleaners in the home as well as in other industrial processes such as the production of medicines, dyes and pigments, fertilizers, and explosives.
In contrast, weak acids are chemicals that release only a tiny fraction of H+. These chemicals tend to be more complex, with more atoms; so, they don’t split apart the way that strong acids do.
They keep a tighter hold on their H+ ions. The most well-known weak acid is acetic acid, the acid in vinegar (5% acetic acid). And just like vinegar, most weak acids have a sour taste.
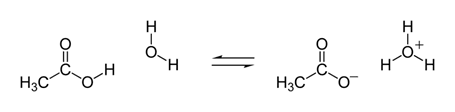
Figure 1: A small fraction of acetic acid releases an H+ to water (H2O) giving H3O+ and a negatively charged acetate ion.
Most weak acids are safe to use as long as they are highly diluted with water. But full-strength concentrated weak acids can still be dangerous.
We regularly use weak acids in cooking and cleaning.
Acetic acid is used as a condiment in food and for pickling vegetables such as cucumbers (pickles). It’s used to descale coffee machines and water coolers. It also has industrial applications in the production of perfumes, dyes and pigments, paints, adhesives, and photography.
Lactic acid is another common weak acid. It’s used as a food additive and added to sour milk products to make yogurt. It’s also a primary metabolite in our bodies. It builds up in our muscles during exercise to cause a burning feeling.
And we can even add water to the list above. Because water on its own can split and release H+, it’s technically a weak acid too.
Bases are chemicals that increase the amount of OH–, making the solution more alkaline. And like acids, we apply the same three models to describe how bases can increase OH–.
Arrhenius defined a base as a chemical that “releases” OH–. This definition, however, doesn’t account for bases that don’t directly release OH– yet, increases OH–. Thus, Brønsted-Lowry defined bases as H+ acceptors, since bases can increase OH– by “accepting” H+ from water. Lastly, Lewis defined bases as chemicals that can donate electrons, like the negatively charged OH–.
Similar to acids, chemicals that dramatically increase the concentration of OH– ions are strong bases, while those that increase it less are weak bases.
The most common strong base is sodium hydroxide (NaOH) otherwise known as lye (or caustic soda). Like a strong acid, it completely splits into OH– and Na+ (think of sodium from table salt). Strong bases require safe handling, as they can cause severe chemical burns.
Sodium hydroxide is used in the strong drain- and oven-cleaners in the home. It’s used in food production for softening olives, making pretzels and bagels, chocolate and cacao production, as well as peeling fruits and vegetables. It’s also used in making soap.
Weak bases add very little OH–. The most common weak base is ammonia (NH3). But ammonia doesn’t have OH– ions to release (hence, the Brønsted-Lowry model).
Instead, the NH3 accepts H+ from water, increasing the concentration of OH– ions. And in the same way that H2O becomes H3O+ when an acid releases an H+; when ammonia accepts a H+, it becomes NH4+.

Figure 2: Ammonia accepts H+ from water to give OH– and an ammonium ion.
Ammonia is a well-known base with a strong pungent odor (smelling salts). It’s most commonly used in cleaning products such as glass cleaners and antimicrobial products. Industrially, ammonia is used in fertilizer production, refrigeration, waste management, and plastic and paper production.
Mixing acids with bases will neutralize both. Acids release H+ and bases release HO–. When put together, they make water (H2O). For example, mixing HCl and NaOH will leave a solution with H2O, Na+ and Cl–. In other words – a glass of salty water!
pH is how we measure the acidity, or alkalinity of a solution. It tells us the concentration of H+ dissolved in water. Mathematically, pH = -log[H+], where [H+] is the concentration of H+. But unless you love math, it’s usually easier to think of the concentration.
For example, if the concentration of H+ was 0.001 Molar (M) or 10-3, then the pH is 3. Likewise, if the concentration is 0.0000001 or 10-7, then the pH is 7. More H+ gives a lower pH reading. A base will reduce the H+ concentration to even lower amounts. An H+ concentration of 10-10 in a basic solution will read a pH of 10.
Acids and bases are commonly used by Mother Nature, herself. The most well-known are the acidic gastric juices in our stomachs. But, not all systems in our bodies work at the same pH levels.
Different systems maintain very different concentrations of H+ and OH–. Our stomachs operate at pH 1.5 to 3.5 (more acidic), while our blood is kept very tightly controlled between pH 7.35 and 7.45 (more alkaline).
And, one critical use of acids is the flow of H+ across cell membranes, which is used to create the very energy we need to live and breathe.
As water is important to our world, so are acids and bases. Chemicals that modulate the acidity and alkalinity of water (pH) are useful for cooking and cleaning in the home. They support the modern world as industrial tools for many applications. And they are vital for all life at a fundamental level.
