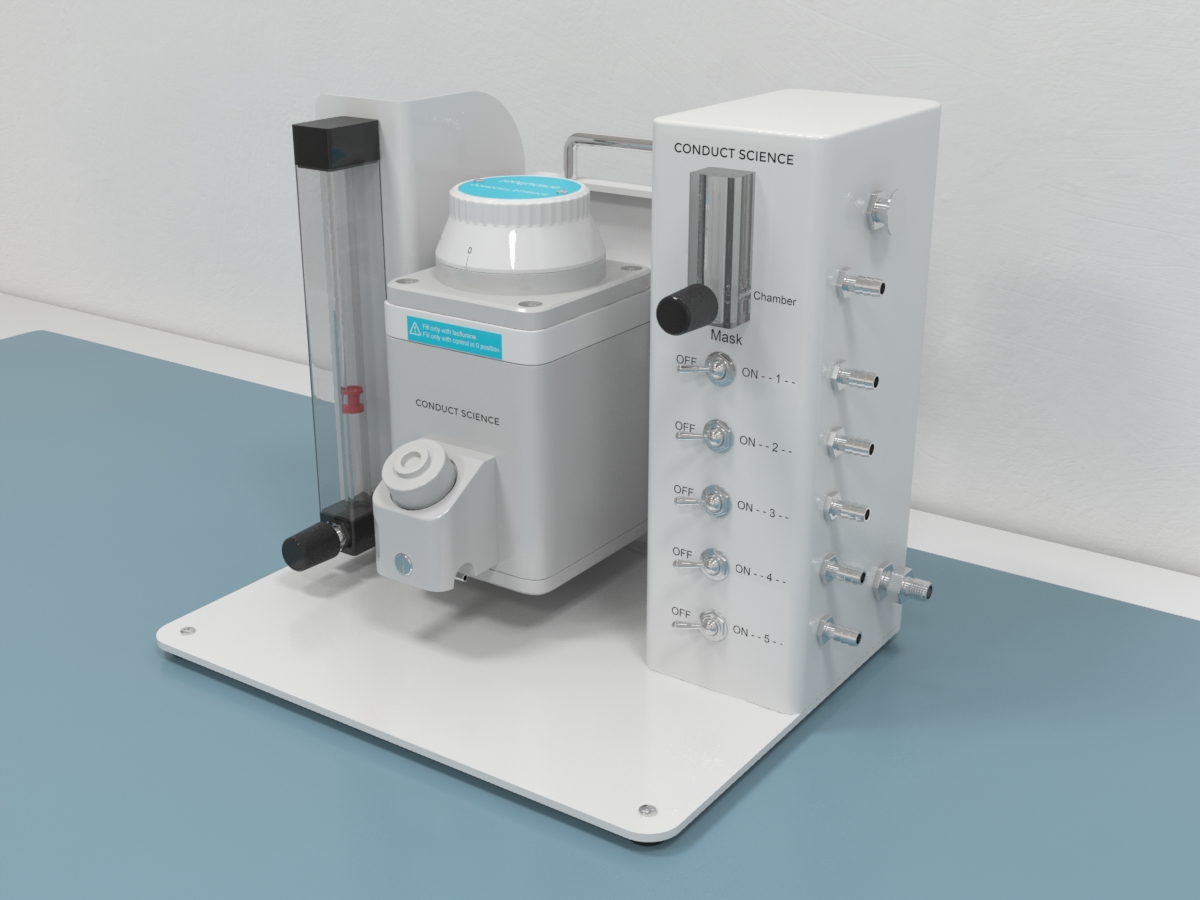
Our Anesthesia Categories
-
$3,990.00
-
 $2,099.00 – $2,199.00
$2,099.00 – $2,199.00 -
 $119.00
$119.00 -
$769.00
Documentation
Introduction
Anesthesia is defined as a temporary state of insensibility or a loss of consciousness. Insensibility can be limited to a region usually by injection of drugs that interfere with the local or regional activity of the nervous tissues (local/regional anesthesia). However, in laboratory practices of anesthetization of animals, general anesthesia is usually preferred. As opposed to local or regional anesthesia, general anesthesia results in loss of consciousness, analgesia, suppression of reflex activity, and muscle relaxation. These features of the latter allow researchers and investigators to undertake surgical procedures with precision, without causing any distress to the animals. Often general anesthesia is induced by inhalation or injectable agents or using a combination of these methods.
Anesthesia administration methods and selection of the agent are usually based on the requirements of the procedures or investigation, species, age, and background of the animal being used. Though the use of an inhalation agents is a popular method of anesthesia induction, animals can be anesthetized by intravenous, intramuscular, or subcutaneous injections and oral or rectal liquid anesthesia. Although a single anesthesia agent can achieve all the features of general anesthesia, usually a combination of agents is used to create the overall effect. The primary advantage of using a mixture of agents is that undesirable side effects of anesthetic agents can often be minimized.
Laboratories often utilize anesthesia machines to administer and maintain anesthesia. The system comprises vaporizers, ventilators, an anesthetic breathing circuit, a waste gas scavenging system, and pressure gauges. The anesthesia is delivered to the animals from the machine via a face mask, a nasal tube, or an endotracheal tube. The system uses compressed gases that are monitored for their flow and pressure rate using flow meters. These gases make their way through the flow meter to a vaporizer where they are transformed into vapors. The vapors then pass on through the breathing system and finally to the subject. Apart from the delivery system, several other instruments are used to ensure adequate airway management and monitoring of the subject throughout the anesthetization process and anesthesia.
Monitoring processes are crucial to ensure the success of the anesthesia procedure. During anesthetization, monitoring of the animal allows for detection of ineffective anesthesia and prevention of injury. The monitoring process should be continued post-anesthesia to ensure proper recovery and to discover any issues that may interfere with the investigatory requirements. Appropriate recovery care is also an essential part of the process. This approach improves the quality of the research while also ensuring animal safety and welfare.
Performing appropriate and effective anesthesia procedures is critical for successful experimental design. Anesthesia has profound effects on the animal’s consciousness and sensation. It enables the experimenters to perform surgical and experimental procedures accurately and with minimal distress to the animal. Achieving adequate anesthesia can be a complex process. Hence, it is important that the anesthetic agents are carefully selected, and appropriate measures are adopted to minimize the unwanted side effects of anesthesia and surgery.
History
Efforts to induce a general state of anesthesia can be traced back to as early as 4000 BCE. Prior to the development of modern anesthesia, surgical treatments were often avoided by people due to a lack of pain management. Scientific discoveries of the late 18th and the early 19th centuries eventually led to the development of modern anesthetic techniques. By the 20th century tracheal intubation and other airway management techniques had become quite common due to the greatly improved safety and efficacy of general anesthesia.
The use of anesthesia in animals was delayed largely due to the misconception that anesthesia induction in animals was unnecessary and painful. However, this ideology was overcome, and veterinary anesthesia became an integral part of animal-based research and investigation procedures. In the 1800s, the potential anesthetic properties of nitrous oxide were suggested by Sir Humphrey Davy which was eventually demonstrated twenty-four years later by H. H. Hickman who mixed nitrous oxide with carbon dioxide to alleviate pain in dogs during surgery. Other drugs and compounds to induce anesthesia were also evaluated in animals. This included diethyl ether, which was extensively used by C. T. Jackson, and chloroform used by Flourens in 1847. In the United States, Dadd (Dadd, 1854) became one of the first advocates of humane treatment of animals and promoted the use of veterinary anesthesia in veterinary surgery. By the end of the 19th century, different routes for anesthetic administrations such as intravenous anesthesia, rectal anesthesia, and intraperitoneal injection were discovered and minimally evaluated. Though the 19th century saw a promising advancement in local analgesic techniques, it wasn’t until well into the 20th century that general anesthesia and humane surgery were adopted into veterinary practices. It can be said that the true beginning of modern era veterinary anesthesia was initiated by the establishment of anesthesia specialty colleges within North America and Europe in the last three decades of the 20th century
Up until the late 19th century, anesthesia delivery did not require a specialized anesthesia machine. However, the introduction of pressurized oxygen and nitrous oxide cylinders required new systems for mounting and delivery. In the year 1917, Henry Edmund Gaskin Boyle designed a continuous flow anesthetic machine based on the American Gwathmey apparatus of 1912. Since its initial design, Boyle’s machine has seen many improvements and additions to enhance its functions of oxygen delivery, accurate mixing of anesthetic gases and vapors, patient ventilation, and minimization of anesthesia-related risks to patients and staff.
The continuous advancement and investigations in veterinary anesthesiology have revolutionized biomedical research. The expansive research in the field has led to better treatment of laboratory animals and the creation of pain-assessment scales and therapeutic guidelines.
Apparatus and Equipment
The process of anesthetization uses a combination of different equipment and instruments to ensure an effective and safe procedure. The apparatus and equipment can be categorized based on their use in the anesthetization process.
Anesthetic Components
The main component of the anesthetization process is the anesthesia machine. The system ensures the delivery of the anesthetic agents at constant pressure. The anesthetic machine is comprised of a gas compressor, a regulator, a flow meter, a vaporizer, an anesthetic breathing system, and a waste gas scavenging system.
Since compressed gases are used with these machines, an appropriate gas delivery hose system must be used to avoid mixing and leakage. The gas supply hoses are flexible and color-coded to ensure no mixing of outlets occurs. One end of these pipes has a Schraeder probe while the other end has a gas-specific threaded connector. The Schraeder valves use a unique collar indexing system with a unique diameter to fit a matching recess on the gas outlet. A pressure-reducing valve ensures that the gases are delivered at the required low pressure. Another component of the anesthetic machine used to control and assess the rate of flow of the pressurized gases is the flowmeters. In modern anesthesia machines, the flowmeters are tapered glass tubes that consist of a bobbin or a ball to measure the flow rate. The flowmeter system consists of a control valve and flow meter sub-assembly. A needle valve is used as the control value and is responsible for controlling the flow of gas passing through the flow meter. Compared to the ball flowmeters, the bobbin flowmeters provide an accurate reading. In the older system, a Turret-type flowmeter can be seen. These flowmeters have the gas flowing out from the bottom of the instrument.
The vaporizers system in the anesthetic machine is used for controlling the vaporization of the liquid anesthetic agent and delivering a reliable concentration to the animal. The system is composed of a concentration control dial, a bypass chamber, a vaporizing chamber, a filler port, and a filler cap. The vaporizer delivers the gas by considering the varying ambient temperature, fresh gas flow, and agent vapor pressure. Vaporizers are designed to be used with specific anesthetic agents and are equipped with filling systems to enforce the same. Filler tubes are agent-specific and fittings on the vaporizer and the collar of the bottles, too, are specific to the agent. This precaution is built into the design to prevent the mixing of the anesthetic agents. Vaporizers can be classified based on their method of output regulation and method of vaporization. Modern vaporizers operate by splintering a small proportion of the fresh gas flow with concentrated anesthetic and then mixing the vaporized gas with the main gas flow. In clinical research, precision vaporizers are often used due to their accurate selection of final concentrations. Another type of vaporizer used is the low-resistance vaporizer.
The breathing circuit of the anesthetic system is responsible for delivering oxygen, delivering anesthetic gases, assisting ventilation, and removing exhaled carbon dioxide. Anesthetic breathing circuits are divided into re-breathing and non-rebreathing systems. Rebreathing circuits permit recirculation and reuse of expired oxygen and anesthetic vapors, making them more economical than non-rebreathing systems. Unlike non-rebreathing circuits, heat and moisture in the rebreathing circuits are preserved. On the other hand, non-rebreathing circuits offer less resistance, less mechanical dead space, and rapid manipulation of the depth of anesthesia by adjustment of the fresh gas inflow. The selection of the type of circuit depends on factors such as tidal volume, minute volume, circuit size, and species. The tidal volume is calculated as the volume of gas drawn in with each breath by the animal. The minute volume is calculated as the product of the tidal volume and the respiratory rate. Though the minute volume is the volume of gas drawn into the respiratory tract in 1 minute, it is not always equivalent to the gas needed to be delivered to the animal by the breathing system. For animals that weigh less than 7kg, non-rebreathing circuits are recommended.
Different types of breathing circuits offer varying degrees of resistance to breathing and differ in dead space volumes. Breathing circuits that have high resistance cause the animal to put in greater respiratory effort. Breathing exertion can lead to respiratory muscle fatigue, reduced respiration, and increased oxygen. Among breathing circuits, the Open Breathing System is the most commonly used in anesthetic machines. These systems are suitable for large animals since it relies on high gas flow to avoid rebreathing of exhaled gases. However, they can be used for smaller animals with the help of concentric face masks or low flow masks combined with a passive scavenging system or a down-draft table to remove waste anesthetic gases. An advantage of these systems is that they allow manual compression in case assisted ventilation is required. On the other hand, the Closed Breathing System relies on soda-line canisters to absorb expired carbon dioxide. Due to the lower fresh gas flows used in these systems, they are often used for large animals. Among Closed Breathing Systems, the Circle System which uses two unidirectional valves to control the flow of gas is the most popular circuit in use. Although these systems are generally used for large animals, a lightweight disposable version with low-resistance valves has gained popularity in the anesthetization of small animals. The advantage that the Closed Breathing circuit has over the Open Breathing circuit is that they conserve heat and moisture. A Semi-closed Breathing System allows some of the rebreathing of the expired gases while no carbon dioxide absorption occurs.
Another type of breathing system is the T-piece System. This system provides low-resistance and low-dead space and is often used in infants and small animals. The system was first described by Ayre in the year 1937. The system uses a T-shaped tubing of which one end is connected to the subject while the other end is attached to a length of tubing to serve as a small reservoir for anesthetic gases. This system reduces the required gas flow to about twice the subject’s minute volume without rebreathing. The T-piece can also be constructed using an a Luer adaptor ‘Y’ and plastic tubing for smaller mammals. A modification of the T-piece is the Bain Coaxial System. In this circuit, the fresh gas inflow tubing runs inside the reservoir limb. The system has a lightweight design that reduces the pull on the endotracheal tubes and the possibility of accidental extubating. The functioning of the Bain system is similar to the T-piece system; During expiration exhaled gas fills the reservoir tube, and during the pause, before the next inspiration this is replaced with fresh gas provided the fresh gas flow is adequate. Magill Breathing System is another circuit that uses a three-way T-tube connected to a fresh gas outlet, a breathing bag, and a reservoir tube. The continuous flow of fresh gas flushes any remaining carbon dioxide-rich alveolar gas down the breathing system during the pause before the next inspiration. However, in small subjects (10 kgs), the system imposes a significant resistance to expiration. Additionally, the typical dead space of the system represents a significant portion of the tidal volume of the animal.
Regardless of the choice of the anesthetic breathing system, a face mask, a nasal tube, or an endotracheal tube are needed to connect it to the animal. Veterinary face masks are usually cone-shaped, and the sizes vary depending on the animal they are being used on. Face masks are selected based on snug fit while ensuring they do not obstruct the mouth or nose of the animal. Small, transparent masks fitted with flexible rubber diaphragms prove to be useful in a large array of animals and birds. The snug fit ensures that the masks are not too large for the animal and prevent the re-inhalation of expired gases. Face masks that incorporate waste anesthetic gas removal (Hunter et al., 1984) are ideal for preventing exposure-related hazards to the personnel.
Apart from veterinary face masks, endotracheal tubes are also often used in the anesthetization of animals. These tubes reduce anatomical dead space and allow the performing of assisted or mechanical breathing procedures when required. The selection of the endotracheal tube size is dependent on the size and weight of the animal and varies for every species and individual. Endotracheal tubes are available in different constructs such as silicone rubber, red rubber, and PVC. However, PVC, polyethylene, and polyurethane are more commonly used due to their transparent nature as opposed to the opaque red rubber. Correct placement of these clear tubes can be evaluated based on the condensation appearing with each breath the animal takes. On the other hand, silicon material endotracheal tubes are translucent and are relatively resistant to kinking. Endotracheal tubes with a wire coil armor reinforcement are frequently used when there is a possibility of excessive flexing of the head and neck of the animal. The wire coil prevents the tube from kinking while still providing sufficient flexibility. However, these tubes should not be used in an MRI. Endotracheal tubes tend to have a preformed curve to allow better visualization of laryngeal opening during intubation. The tubes have a beveled distal end and may also have a small opening opposite the bevel called the Murphy eye as an additional path for the gases in case the bevel is occluded. Primarily, endotracheal tubes are available as cuffed and uncuffed tubes. The cuffed tubes provide a seal around the tube to prevent leakage of anesthetic gases and aspiration of oral secretions or gastric contents. These tubes are available in two variants; low-volume, high-pressure cuffs and high-volume, low-pressure cuffs. Low-volume, high-pressure cuffs provide a narrow seal at the tracheal wall and require high pressure for inflation, while the latter type of cuff allows a greater area of tracheal contact and requires low inflation pressure.
The tracheal placement of the endotracheal tubes is assisted by laryngoscopes. The modern-day laryngoscope is equipped with battery-operated lights and a set of blades to allow a clear view of the larynx during the insertion of endotracheal tubes. The selection of the type and size of the blade is dependent on the species and the animal’s size. The handle of the laryngoscope not only houses the battery but also plays an important role as a counterbalance for the blades. The two popular laryngoscopes in use are the Miller Laryngoscope, which uses a straight blade, which uses a curved blade. The Miller blades may be more useful than MacIntosh blades in subjects with short, thick necks, higher positions of larynxes in the neck, big tongues,s or those that are obese.
Laryngeal Masks and V-gel Airways provide an alternative to endotracheal tubes for maintenance and establishment of clear airways. The V-gel Airway device has a soft, non-inflatable cuff that rests over the larynx. In comparison to the Laryngeal Mask, the V-gel Airway device is considered easier to be placed and causes less tracheal mucosal damage than the endotracheal tubes (Oostrom et al., 2013; Uzun et al., 2015). Another approach to the delivery of anesthesia from the anesthetic machine to the animals is the Anesthesia Induction Chamber. The induction chamber is used for small animals and allows the anesthetization of one or a group of animals at a time.
The final important component of an anesthetic machine is the Gas Scavenging System. Scavenging machines are used in the collection and removal of waste anesthetic gases vented during the anesthetization processes. The practice prevents a variety of hazards associated with anesthetic gas pollution of the operating area including risks to personnel resulting from chronic exposure to low levels of some inhalational anesthetics. Scavenging machines also help monitor the gases and prevent barotrauma to the subject resulting from a change in incoming and outgoing gas flow. Anesthetic gas scavenging machines comprise a gas collection assembly that contains tubes connected to adjustable pressure limiting (APL) valve and vent relief valve, transfer tubing, scavenging interface, gas disposal tubing (used for carrying gas from the interface to disposal assembly), and gas disposal assembly. The scavenger interface plays a critical role in protecting the breathing circuit from excessive positive or negative pressure. Waste anesthetic gas systems can be classified as either active scavenging machines or passive scavenging machines. Active scavenging systems make use of fans or vacuum pumps to create a continuous low pressure in the interface to draw the waste gases into the disposal systems, while passive scavenging systems utilize the pressure generated in the breathing circuit to exhaust the gases to the interface. The difference between the two types is that the active systems protect the subject’s airway from suction and build-up of positive pressure while the passive systems protect from the build-up of positive pressure only. Waste anesthetic gas evacuation systems can also be categorized based on the type of interface, which can be either open or closed. The open interface design is often seen in the newer gas machines and is open to the room and relies on continuous suction. Closed interfaces, however, make use of valves and are often seen in older machines.
Corrugated plastic pipes are used to connect most components of the anesthetic machine. These tubes are kink resistant, extremely flexible, and lightweight. Further, they are tough and resistant to chemical attacks. Breathing tubes are used to connect the system to a face mask, laryngeal mask airway, or tracheal tube to support breathing or the administration of anesthesia. The Luer taper is a standardized system of fluid fittings used to connect male-taper fitting and female-taper fitting on pipes and systems. These connections are available in locking and slipping varieties. The locking variety, also known as the Luer lock connectors, relies on the tabbed hub on the female fitting which screws into threads in the sleeve on the male fitting to create a secure join. The connectors are further divided into one-piece Luer lock and two-piece Luer lock or rotating collar Luer lock. The one-piece connector is a single mold Luer lock that achieves locking by rotation of the entire Luer connector or system, while the two-piece Luer lock achieves locking by the rotation of the free rotating collar with threads. Slip tip, or the Luer-slip, fittings are held together by friction.
Anesthesia Management and Monitoring
Monitoring of the anesthetic equipment and the animal’s vital signs enables the detection and correction of issues that may lead to inefficiency and complications during the procedures.
Respiratory Systems
Deterioration of respiratory functions can be detected by monitoring the reservoir bag in the breathing system or with the help of an oesophageal or conventional stethoscope. Additionally, Respiratory Monitors can be used for monitoring respiratory rate. These monitors are also available for animals that weigh as much as 300 g. Alternatively, Pressure Sensors can be used for the same task. Pressure Sensors rely on chest movement to monitor respiratory function. Further, Wright’s Respirometer can be used in assessing the tidal and minute volume of the animal. The Respirators are commonly used to evaluate the appropriate functioning of mechanical ventilators in delivering the intended volume of gas. The selection of these devices should be based on the size and species of the animal.
Pulse Oximeter and Capnograph are used in the monitoring of hypoxia and hypercapnia respectively. Pulse Oximeters detect changes in the absorption of light across tissues (such as the tongue or digit) to measure the percentage oxygen saturation of arterial blood. Further, the variation of this signal is also measured with each heartbeat to calculate the heart rate. Capnograph, on the other hand, enables monitoring of the end-tidal concentration of carbon dioxide in the exhaled gases. The graph obtained also allows observation of changes in respiratory rate and pattern. The Capnograph aids the anesthetist by alerting them of the abnormal build-up of carbon dioxide and rebreathing of exhaled gases. Based on their placement in the anesthetic delivery system, Capnograph can be either side-stream systems or main-stream samplers. Side-stream systems are designed to sample expired gases from a tube placed close to the endotracheal tube while main-stream samplers have the carbon dioxide sensor placed directly in the breathing system.
Another instrument used in the monitoring of the respiratory system is the Blood Gas Analyzer. These analyzers measure the partial pressure of oxygen, carbon dioxide, and pH of the blood. They also calculate the blood bicarbonate concentration and the base excess. Although rarely used in laboratory animals, transcutaneous oxygen and carbon dioxide monitors can be used to approximate concentration of the arterial carbon dioxide and oxygen (Ramos-Cabrer et al.,
2005; Sahbaie et al., 2006; Barter & Hopper, 2011).
Other measures to ensure the appropriate functioning of the delivery system and the safety of the animal include the use of pressure relief valves to avoid over-pressurization from the vaporizer and flowmeter, emergency oxygen flow that is operated by a spring-loaded key, and an oxygen failure alarm to alert the anesthetist or investigator of dropping oxygen pressure.
Cardiovascular System
Systemic arterial pressure can be measured directly and indirectly in animals. Direct measures require the invasive procedure of arterial cannulation. A sphygmomanometer can be used to provide a non-invasive, indirect measure of arterial pressure. Another instrument that can be used to detect arterial pressure is a Doppler probe. The probe is coupled with an inflatable cuff and a pressure sensor to provide the measure of arterial pressure in a wide range of animals. Most commonly used automated instruments make use of oscillometric technique and take readings at a minimum interval of a minute. For measuring central venous pressure, a catheter is inserted into the jugular vein, and the cannula is connected to a water manometer to record the central venous pressure. In smaller animals, Electronic Pressure Transducers are preferred.
An electrocardiogram (ECG) used in people can usually be used for large animals to monitor the electrical activity of the heart. For smaller animals, purpose-designed instruments are available. ECG electrodes can be satisfactorily used in large animals; however, for small rodents, needle electrodes are usually used.
Body Temperature
Temperature monitoring can be done using a glass clinical thermometer or an electronic thermometer. Glass clinical thermometers require constant adjustments and does not cover temperatures lower than 35°C. Thus, a sophisticated electronic thermometer with temperature probes of different designs is preferred.
Anesthetic Equipment Function
Disconnections in the anesthetic breathing system can be limited by using a safe-lock type connector. Thermistor-type apnoea alarms can be used to alert the anesthetist or the investigator of a disconnection in the breathing system. Pressure monitors and oxygen analyzers can also be used along with alarms to detect falling pressure of disconnections.
Infusion pumps with warning systems are essential in an anesthesia induced by continuous intravenous infusion. These systems detect pump failures and empty infusion reservoirs or syringes. Newer infusion systems are microprocessor-controlled and can be fitted with an array of devices to alert the anesthetist or the investigator of malfunctions.
Post-operative Care
Recovery areas are an important part of the anesthetization process. Animals should be allowed to recover in separate areas from surgical areas. Animal incubators provide control of temperature and administration of oxygen. Alternatively, specialized warming racks may also be used for small animals. For larger animals, a recovery pen can be used. The bedding and nesting material of these areas can be synthetic materials similar to sheepskin, a shredded paper that will not stick to the animals (small animals) wounds or orifices, or deep straw beds (small ruminants and pigs). Towels or blankets can also be used in the recovery areas.
Temperature control can be assisted with the use of warming lamps or heating pads. Using forced air warming systems around a standard rodent cage can also be used to maintain ambient temperatures (Rembert et al., 2004).
Veterinary Anesthesia Agents
Veterinary anesthesia ensures that the animals are not caused any distress and remain immobile during the surgical procedures. This allows researchers and investigators to perform experiments and surgeries with precision and in a humane way. The selection and depth of anesthesia are dependent on the procedures that are to be undertaken, the size, the age, and the species to which the animal belongs.
Certain agents induce sleep in the animals but do not have any specific analgesic properties. These are known as hypnotics. However, at high doses hypnotics are known to produce general anesthesia. Protocols that make use of hypnotic agents also use an analgesic agent to relieve pain. The way insensibility and unconsciousness are induced vary depending on the type of anesthetic and hypnotic agents used.
Certain investigatory procedures may require the use of neuromuscular blocking (NMB) drugs in addition to anesthesia. The use of neuromuscular blocking (NMB) drugs may be needed, particularly for cardiothoracic and neurophysiological procedures, to eliminate spontaneous muscle movements that can potentially interfere with the quality of the data collected.
Inhalant Anesthetic Agents
Inhalant agents are used with anesthetic machines and are usually delivered in combination with the fresh gas flow or carrier gases. The selection of the anesthetic concentration is based on the agent’s boiling point; a lower boiling point allows the agent to be easily vaporized, thus, allowing higher concentrations to be delivered. Further, the apparent potency and efficacy of the agent can be determined by its minimum alveolar concentration (MAC)50 value. Drugs with low MAC value and low boiling point tend to be very potent. Therefore, it is critical that great care is taken when they are used, to avoid over-dosing the animal.
The anesthetic agent’s potency along with the concentration of anesthetic used and the blood-gas partition coefficient plays an important role in the speed of anesthesia induction and the rate of recovery. Agents with high blood-gas partition coefficients result in a slower rate of anesthesia induction and a slower rate of recovery. Another factor to be considered while selecting an inhalant anesthetic agent is the possibility of internal metabolization. The internal metabolization of certain drugs within the animal can influence certain investigatory data.
Isoflurane
- Produces very rapid induction and recovery from anesthesia.
- Easy and rapid alteration of the depth of anesthesia.
- Non-irritant, non-explosive and non-flammable.
- Undergoes minimal biotransformation compared to other agents.
- Almost completely eliminated with exhaled air.
- Moderate respiratory and cardiovascular depression.
- The pungent odor is not a significant issue in most species apart from rabbits and guinea pigs.
Sevoflurane
- Produces very rapid induction and recovery from anesthesia (more than Isoflurane).
- Easy and rapid alteration of the depth of anesthesia.
- Less pungent odor than other agents. However, it is not tolerated well by rabbits and guinea pigs.
- Significant toxicity is highly unlikely.
- Relatively expensive.
- Unstable in the presence of soda lime.
- Renal injury can result from the breakdown of products. However, under normal conditions, the concentration of breakdown products is very low (O’keeffe and Healy, 1999).
Desflurane
- Produces the most rapid induction and recovery from anesthesia.
- Undergoes the least degree of metabolism (Kolbin, 1992).
- Relatively non-irritant.
- Relatively expensive.
- Requires a pressurized, temperature-controlled vaporizer due to the very low boiling point.
Halothane
- Produces rapid induction and recovery from anesthesia.
- Easy to vaporize.
- Non-irritant, non-flammable and non-explosive.
- Produces cardiovascular depression.
- Produces moderate hypotension at surgical levels of anesthesia.
- Causes dose-dependent depression of respiration.
- Some hepatic metabolism occurs (Wood and Woad, 1984).
- Marked liver microsomal induction may follow anesthesia (Wood and Woad, 1984).
Enflurane
- Produces rapid induction and recovery from anesthesia.
- Easy and rapid alteration of the depth of anesthesia.
- Non-irritant, non-explosive and non-flammable.
- Largely eliminated via lungs.
- Very little is metabolized in the liver (Mazze et al., 1977).
- Produces depression of cardiovascular and respiratory depression comparable to Halothane anesthesia.
Methoxyflurane
- Potent analgesic effect and some post-operative analgesic effect.
- Non-irritant, non-flammable, and non-explosive in air or oxygen.
- Produces some cardiovascular and respiratory depression (generally less than Halothane).
- Metabolism of the drug results in fluoride ion release that may cause renal damage which is significant during prolonged periods of anesthesia (Murray and Fleming, 1972).
- Is an excellent agent for induction and maintenance of anesthesia in neonatal animals.
Nitrous Oxide
- Produces minimal cardiovascular and respiratory system depression.
- Very low anesthetic potency. Cannot be used on its own to induce anesthesia or unconsciousness in most species.
- Prolonged anesthesia (>6 hours) can cause bone marrow depletion due to a reaction with vitamin B12 (vitamin depletion).
- Efficient for reducing the required concentration of other potent anesthetic agents when used in combination.
- Following prolonged administration, 100% oxygen should be administered to prevent diffusion hypoxia.
Injectable Anesthetic Agents
Another method of anesthesia induction is by administration of drugs via injections. Injectable agents can be short-acting or long-acting anesthetics. With the former variety, it is preferable that they are administered in incremental doses so that the effect on the depth of anesthesia is seen rapidly and adjusted readily. Other routes of administration apart from the intravenous route are possible. However, the depth of anesthesia is likely to vary less predictably. Care must be taken when using short-acting agents. Alternatively, long-acting anesthetics can be used instead of repeated doses of short-acting anesthetics.
Barbiturates
Pentobarbital
- Administration can be done either by intravenous or intraperitoneal injection.
- Can be used in a wide range of species.
- Produces severe cardiovascular and respiratory system depression.
- Poor analgesic activity.
- Recovery can be prolonged.
- Can be used as a euthanasia agent (local anesthetic recommended).
Thiopental
- Short-acting barbiturate.
- Produces smooth and rapid induction of anesthesia following intravenous injection.
- Can be used in almost all species.
- Poor analgesic activity.
- Causes transient apnoea.
- Is an irritant when injected perivascularly.
- Repeated administration can result in a very prolonged recovery time.
Methohexital
- Produces smooth and rapid induction of anesthesia following intravenous injection.
- Can be used in a wide range of species.
- Poor analgesic activity.
- Causes transient apnoea.
- Muscular tremors frequently accompany recovery. Administration of pre-anesthetic medication is recommended.
- Repeated incremental doses do not unduly prolong the rate of recovery.
Sodium thiobutabarbital
- Long-acting agent.
- Produces smooth and rapid induction of anesthesia following intravenous injection.
- Variable analgesic activity.
Steroid Anesthetics
Alphaxalone/Alphadolone and Alphaxalone
- Produces smooth and rapid induction of anesthesia following intravenous injection.
- Repeated doses have minimal effect on the rate of recovery.
- Non-irritant.
- Alphaxalone and alphadolone mixture has a wide margin of safety in most species (Child et al., 1971; Box and Ellis, 1973).
- Solubilizing agent present in the commercial preparation of alfaxalone/alphadolone promotes histamine release in dogs.
- Mild histamine release also occurs frequently in cats, causing edema of the paws, muzzle, and
- Alphaxalone/alphadolone administration by the intramuscular or intraperitoneal routes has very variable effects.
- Alphaxalone/alphadolone must not be used in conjunction with barbiturates.
Dissociative Anesthetics
Ketamine
- Can be administered by the intramuscular, intraperitoneal, and intravenous routes.
- Produces immobility in most species.
- Causes moderate respiratory depression in most species,
- Increases blood pressure.
- Is an NMDA (N-methyl-D-aspartate) antagonist and is known to prevent sensitization to noxious stimuli during surgery.
- Increases skeletal muscle tone.
- Produces a very variable degree of analgesia.
- Severe respiratory depression is produced in small rodents following high dose administration rates.
- Recovery can be prolonged and may be associated with hallucinations and mood alternations.
- Administration in combination with atropine or glycopyrrolate to reduce excessive bronchial and salivary secretions may be necessary for all species.
- In all species, it may be necessary to use atropine or glycopyrrolate together with ketamine to reduce these otherwise
- Long-term administration of ketamine can result in bladder irritation (Morgan and Curran, 2012).
- Repeated administration of ketamine results in tolerance (Douglas & Dagirmanjian, 1975).
Neuroleptanalgesics
Fentanyl/Fluanisone, Fentanyl/Droperidol, Etorphine/Methotrimeprazine, Etorphine/Acepromazine
- Combinations produce profound analgesia.
- Can be administered by the intramuscular, intraperitoneal, or intravenous routes to most species.
- Mu-opioids (such as naloxone, nalbuphine, or butorphanol) can be used to reverse the effects of these combinations.
- Produce moderate or severe respiratory depression.
- Have a poor degree of muscle relaxation.
- May also produce hypotension and bradycardia.
Fentanyl/Medetomidine
- Produces reliable surgical anesthesia with good muscle relaxation in some species.
- Anesthesia can be completely reversed by the administration of specific antagonists such as nalbuphine or butorphanol along with atipamezole.
- Produces mild to moderate respiratory depression.
- In mouse causes urinary retention.
Hypnotics
Etomidate and Metomidate
- Short-acting hypnotics.
- Produces minimal effect on the cardiovascular system.
- Produces little analgesic effect on its own.
- Prolonged infusion can result in suppression of adrenocortical function (Dodam et al., 1990).
Propofol
- Produces rapid induction of short-period anesthesia.
- Can be used in a wide range of species.
- Produces smooth and rapid recovery that is minimally affected by additional doses.
- Can produce respiratory depression at high doses.
- Rapid administration can result in insufficient analgesia in some species and a short period of apnoea.
Tribromoethanol
- Produces surgical anesthesia in rats and mice.
- Produces good skeletal muscle relaxation.
- Produces rapid recovery (Papaioannou and Fox, 1993).
- Produces moderate degree of respiratory depression.
- Incorrect storage or administration of more than one dose can lead to irritation of the peritoneum.
Chloral Hydrate
- Produces medium duration stable and light anesthesia (Field et al., 1993).
- Produces minimal effect on the cardiovascular system and baroreceptor reflexes.
- Has poor analgesic properties.
- High doses can produce severe respiratory depression.
- A high incidence of post-anesthesia ileus in rats can be observed after intraperitoneal administration (Fleischman et al., 1977).
Alpha-Chloralose
- Produces stable, long-lasting light anesthesia.
- Produces minimal depression of the cardiovascular and respiratory systems.
- Has poor analgesic properties that vary considerably between different species and strains.
- Produces prolonged induction and recovery with involuntary excitement.
Urethane
- Produces long-lasting anesthesia.
- Produces minimal cardiovascular and respiratory depression.
- Carcinogenic in nature (Field and Lang, 1988).
- Produces peritoneal effusion (Severs et al., 1981) and hemolysis.
Protocol
Pre-Anesthesia Protocol
4.1.1 Acclimation
Acclimation period allows the handlers and experimenters to familiarize themselves with the behavior and characteristics of the animals. It also helps return to normal the metabolic and hormonal changes of the animals after being transported (Obernier and Baldwin, 2006).
Ideally, animals should be obtained at least 7 to 14 days before their use in investigations and experiments. During this period, they should be monitored for any signs of illness and records of body weight, growth rate and food and water consumption should be made. These variables enable the understanding of animal’s recovery post-procedure, assessment of pain and normal physiological state. Regular handling of most species habituates them to the procedure and makes more cooperative. Further, habituating the animals to their holding and recovery environments reduces the stress of novelty and social isolation (if animals are singly housed).
4.1.2 Clinical Examination
It is recommended that a general clinical examination is performed on the animals prior to anesthetization. Any abnormality or clinical signs such as the presence of discharges from the eyes or nose, matting of the fur around these regions or soiling of the perianal region with feces may require further investigation and expert opinion. Food and water consumption of the animals should also be monitored on pre-operative days to allow post-operative assessment of normal intake.
4.1.3 Pre-Anesthetic Fasting
The requirement of pre-anesthesia fasting is depended on the species being used and the risk of vomiting. In large animals such as cats, dogs, pigs, primates, and ferrets an 8 to 12 hours period of fasting is recommended to minimize the risk of vomiting during induction and recovery from anesthesia. In ruminants, 12 to 24 hours of pre-anesthetic fasting may help reduce incidences of ruminal tympany or bloat.
In smaller animals such as rabbits and rodents, fasting is usually unnecessary. However, in guinea pigs, 3 to 4 hours pre-anesthetic fasting may be implemented if a large number of the animals in the cohort show signs of retaining food in their pharynx. For gastrointestinal surgery, all species may be required to fast to reduce the volume of gut content. Further, in species that are coprophagic, measures to prevent them from ingesting their own feces must be in place during the fasting period.
Another consideration for pre-anesthetic fasting is the diurnal rhythms of some species. Some species may avoid eating post-operatively until the onset of the dark phase of their photoperiod. Further, pain, surgical stress, or delayed recovery can impact the food and water intake severely. Thus, extreme caution should be practiced in such species if pre-anesthetic fasting is necessary.
A 6 to 12 hours period of fasting in large and medium-sized birds is sufficient to reduce the risk of regurgitation. In smaller birds, a fasting period of no longer than 2 hours should be used to avoid the risk of inducing hypoglycemia. In general, the fasting of reptiles, fishes, and amphibians is generally unnecessary. Further, the fasting of pregnant animals of any species can lead to severe metabolic disturbances that may result in fatality.
The provision of drinking water to all animals should be stopped approximately 60 minutes prior to the induction of anesthesia. In case of reduced fluid intake, vomiting, diarrhea, or hemorrhage, pre-operative fluid therapy should be initiated.
4.2 Administration of Anesthesia
The route of administration is based on the experimenter’s experience with the procedure and equipment, the research protocol, and other practicalities. Commonly, general anesthesia in animals can be produced by either using injectable or inhalational agents or a combination of these methods.
4.2.1 Anesthetic Machine
Anesthetic machines rely on face masks or endotracheal intubation to deliver the anesthesia to the animal. Alternatively, anesthetic chambers can also be used for the process of anesthetization. The selection of the method of connecting the animal to the anesthesia machine is usually based on the experimenter’s comfort level using the equipment and the requirements of the surgery. Another consideration is the management of waste anesthetic gas.
Endotracheal Intubation
Endotracheal intubation is assisted by a laryngoscope. The type of endotracheal tube and laryngoscope used are dependent on the size and weight of the subject. Commercially available endotracheal tubes tend to be excessively long. Thus, it must be cut down to an appropriate length usually to the approximate distance from the external nares to just anterior to the thoracic inlet. An uncuffed endotracheal tube is preferred for small animals. The tubes should be lubricated with a small quantity of lidocaine gel to allow easy passage. Further, cough and swallowing reflexes should be adequately numbed. It is recommended that the animal is administered with oxygen for about 2 minutes before being intubated to slow the advent of hypoxia should the larynx be obstructed. The detailed methodology of endotracheal intubation of different animals can be found here.
The procedure of insertion of the endotracheal tube is performed by resting the animal on its back (generally), with the neck and head flexed. The laryngoscope is then advanced over the tongue towards the pharynx. Care must be taken to extend the tongue and avoid damaging the surface of the teeth of the animal. Curved laryngoscope blades are positioned anterior to the epiglottis while straight blades are positioned posterior to the epiglottis. Following successful laryngoscopy, the endotracheal tube is advanced into the trachea.
Anesthetization of Individual Animals
(Protocol for rodents using a Bain breathing circuit)
- Before beginning with the anesthetization procedure, check all the connections in the system, ensure that the appropriate quantity of all the gases is available and that the parts that need to be replaced are replaced.
- Turn on the oxygen source and adjust the fresh gas flow in the Bain circuit to a rate of 1 L/min using the flowmeter control knob.
- Holding the animal by the scruff, firmly position its nose against the opening of the circuit (nose-cone adaptor or face mask).
- Turn on the vaporizer and adjust the inhalant agent concentration depending on the age and condition of the animal. If using isoflurane, usually the concentration is set to 4 to 5%.
- Adjust the oxygen flow to 0.5 L/min and reduce the concentration of the inhalant agent to a concentration suitable to achieve the surgical plane of anesthesia (isoflurane concentration to 2.5 to 3%) once the animal offers no resistance to manual restraint.
- Monitor the anesthesia depth throughout the procedure.
- Once the procedure is over, shut off the vaporizer and continue to maintain the oxygen flow to the animal until it regains consciousness.
- Shut off or disconnect the oxygen flow.
- Place the animal back in a clean cage, preferably by itself or with similarly anesthetized animals.
- Once the anesthetization procedure is complete, ensure that the oxygen flow has ceased and the control knob on the flowmeter is in the OFF position.
Anesthetization of Group of Animals
(Protocol for rodents using Bain breathing circuit)
- Before beginning with the anesthetization procedure, check all the connections in the system, ensure that the appropriate quantity of all the gases is available and that the parts that need to be replaced are replaced.
- Turn on the oxygen source and adjust the fresh gas flow into the anesthesia induction chamber to a rate of 2 L/min using the flowmeter control knob.
- Place the animals in the chamber and tightly close the lid.
- Turn on the vaporizer and adjust the inhalant agent concentration depending on the age and condition of the animals. If using isoflurane, usually the concentration is set to 4 to 5%.
- When the animal appears to be moderately anesthetized, remove one of them from the chamber and immediately close the lid.
- Connect the removed animal to the Bain circuit’s nose-cone adapter or face mask and open the glass flow.
- Maintain the gas flow in both the nose cone and the chamber at a 2 L/min rates.
- Reduce the concentration of the inhalant agent to a concentration suitable to achieve the surgical plane of anesthesia (isoflurane concentration to 2.5 to 3%).
- Recover the animal by shutting the anesthetic flow to the nose cone or face mask and allowing it to breathe room air. Return it to a clean cage when awake.
- Repeat the process for the remaining animals in the chamber.
- Once the procedure is over, shut off the vaporizer and close the valve to the Bain circuit.
- While keeping the lid closed, flush the chamber by increasing the oxygen flow to 4 to 5 L/min and allowing it to displace the anesthetic gas into the waste anesthetic gas scavenging system.
- Shut off or disconnect the oxygen flow and wait until the flowmeter reads zero.
- Close the flowmeter.
4.2.2 Injectable Agents
Injectable agents can be administered intravenously, intramuscularly, intraperitoneally or subcutaneously. However, intravenous administration produces the most predictable and rapid induction of anesthesia. In comparison to the intravenous route, other routes of administration produce a considerably varying anesthetic effect due to the varying rate of absorption. Further, these routes require large doses of the anesthetic to produce the required effect and due to slow absorption tend to produce prolonged residual drug effect and prolonged complete recovery. An important consideration while selecting an agent is the variation of response among different species, strains, ages, and sexes of animals. Though the simplest method of administration is using syringes, butterfly-type infusion sets, indwelling catheters and infusion pumps can be used to aid the process.
4.3 Pre-Operative Preparation
Pre-operative preparations are made considering the best-suited situation for the researcher and the animal. Usually, a compromise is made between the ideal working situation for research and animal welfare. During anesthesia, animals lose their protective reflexes of the eye which invariably leads to damage to the cornea. Precautions such as using a bland ophthalmic ointment or taping the eyes closed to eliminate damage must be taken. Further, during operative surgeries, there may arise a need to retract the limbs. While binding the limbs of the animal care must be taken to ensure that the limbs are not retracted too far off nor are the limbs tied too tightly; This eliminates the risk of causing tissue damage and peripheral limb edema. The use of elastic bands should also be avoided to tie limbs or abdomen as they can significantly interfere with the respiratory functions of the animal. Also, care should be taken to not put undue pressure on the chest wall or the abdomen of the animal during surgery.
During the operative procedures, it is important that the head and neck remain extended to prevent the obstruction of the larynx by the tongue or the soft palate. Further, the endotracheal tube, if used, must be firmly tied to the animal’s jaw and should be tapped down to prevent dragging on the endotracheal tube and dislodging it. Care must also be taken while repositioning or turning the animal. It is best to temporarily disconnect the animal from the breathing system to prevent kinking of the endotracheal tube.
4.4 Monitoring and Management of Anesthesia
Since the effects of anesthetics vary based on factors such as species, strain and age, and the agent used, monitoring of the process of anesthesia is essential. Further, respiratory system, cardiovascular system and thermoregulatory mechanism depression produced by many anesthetic agents can result in hypoxia, hypercapnia, acidosis, hypothermia, reduced tissue and organ blood flow, making appropriate management of anesthesia a necessity. Apart from these factors, monitoring of the animal’s vital signs during the anesthetized and recovery period will allow the discovery and rectification of any issues that can potentially affect the research outcomes.
4.4.1 Assessment of the Depth of Anesthesia
A combination of measures can be applied to assess the depth of anesthesia in the animal. These measures include analyzing the response to painful stimuli, changes in pattern and depth of respiration, changes in muscle tone, and changes in heart rate and blood pressure. Although not yet widely applied in animal-based research, electroencephalogram (EEG) and sensory or somatic evoked potentials measure used in humans can be used to provide a more sophisticated approach to the assessment of depth of anesthesia.
Responses to Painful Stimuli
In most species, post-anesthesia response to painful stimuli can be assessed by pinching the toes, tail, ears, or nose. Limb withdrawal reflexes can also be used to assess the depth of anesthesia. This reflex can be performed by extending the hindlimb of the animal and pinching the web of skin between the toes. In smaller animals like small rodents, pinching the skin between the toes may be difficult, in this case pinching the tail can be used as an alternative. Animal vocalization or withdrawal of the limb signifies the insufficient depth of anesthesia. In most circumstances, a slight, barely perceptible movement remains indicative of the sufficient depth of anesthesia to perform surgical procedures. In some animals, such as rabbits and guinea pigs, pinching of the ear may also be used to assess the depth of anesthesia.
However, this simple method does not necessarily indicate loss of consciousness. The withdrawal reflexes are primarily mediated by spinal mechanisms; the depth of anesthesia used to repress these responses generally tends to be greater than that needed for the loss of consciousness. Pain stimuli response-based assessment of depth of anesthesia may thus result in anesthesia that is more than required to produce loss of consciousness and amnesia (Antognini et al., 2005).
Alterations in Eye Reflex
Palpebral and corneal reflexes can also be used to assess the depth of anesthesia. However, alterations in eye reflexes vary between species and tend to be difficult to assess in small rodents. Further, in small animals, the loss of eye reflexes may be indicative of a very deep plane of anesthesia. Additionally, the position of the eyeball, the degree of pupillary dilation and occurrence of nystagmus may also aid with an assessment of the depth of anesthesia. Regardless, alterations in eye reflexes significantly vary with species and agent used. Thus, it is advisable that eye reflex assessment is used in combination with other assessments to attain a reliable measure of the depth of anesthesia.
Alterations in Cardiovascular and Respiratory Functions
Generally, different anesthetics produce varying levels of depression in the respiratory and cardiovascular systems. Respiratory depression manifests itself with a change in rate and depth of breathing and the pattern of breaths, while cardiovascular depression results in a fall in arterial blood pressure (either with a fall or a rise in heart rate). Since depression produced is highly variable, using this measure for the assessment of the depth of anesthesia is not ideal and can be dangerous to generalize.
4.4.2 Monitoring of Body Functions
Basic clinical observations such as noting the color of the mucous membranes, the pattern and rate of respiration, and the rate and quality of the pulse provide important insights into issues that may arise during anesthesia. These observations along with observations made using sophisticated devices allow detection and correction of any problems before they become irreversible. This is particularly important to ensure the humane treatment of the animals and the quality of the research and the research data.
4.5 Post-Operative Care
Post-operative care is not just necessary for accurate and reliable research but also to ensure the humane treatment of the animals. Good anesthesia practice involves good recovery care practices. Post-operative care must be based on the operative procedures and the species involved.
A major concern during the post-anesthesia period is the management of pain. Maintenance of effective analgesia should be based on careful assessments; Analgesic regimen should be selected depending on the species, the characteristics of pain (nature, duration, and intensity) and the specificity of the research procedure. Based on the pain assessment of the animal, they may arise a need to modify the regimen in place to offer better pain control.
4.5.1 Recovery Environment
Post-operatively, hypothermia can be a serious issue in recovering animals. An appropriate recovery environment is essential to combat this and other physiological issues. Recovery environments should be maintained separate from the operative area and should provide a warm and quiet space for the animals to recover. For smaller animals heating pads can be used to create a warm space in acrylic cages. Incubators used for human neonates tend to be a better option than commercially available animal incubators for small animals. Warming lights can be used for both small and large animals to create ambient temperatures in the post-operative periods. Alternatively, forced warm air systems can be used to regulate the environment’s temperatures.
Ideally, adult animals should be kept at 27–30°C and neonates at 35–37°C temperatures post-operatively. The temperatures should then be maintained at 25°C for adults and 35°C for neonates once they have recovered from the major depressant effects of anesthesia. It is important that the animals are not overheated during the recovery period. To ensure this, body and environment temperatures should be taken from time to time.
The choice of bedding used is also important. The materials used should not cause discomfort to the animal by sticking to wounds, animal’s orifices, or to their eyes, nose, or mouth. Synthetic bedding such as sheepskin provides an ideal bedding material that is washable, autoclavable, and extremely durable, and comfortable for the animal. Paper shavings that meet the criteria can be used in the recovery areas of small animals. Deep straw beds are also ideal bedding for small ruminants and larger animals.
4.5.2 Nursing
Human contact can be beneficial in the recovery process of animals provided that the animals have been familiarized with the staff during the pre-operative period. Since animals’ response to human contact varies and, in some animals, can induce stress, appropriate measures must be taken to ease the situation.
Post-operatively animals may be reluctant to eat. In this case, hand feeding the animal warmed food may encourage them to eat thus improving their appetite. Certain animals such as cats, dogs, and pigs, respond well to stroking and familiar personnel. Further, animals that have not resumed their normal grooming habits usually greatly appreciate grooming by the staff. These techniques, among others, can have a significant impact on the recovery of the animal.
In all species, immediate post-operative care may be needed which should be followed by observations every 1 to 4 hours for the first 8 to 12 hours. It is important that they are checked at least once every day and attention is paid to cleaning their eyes, nose, and mouth. Post-operative care should also include monitoring of body weight and checking of wounds and surgical implants. Keeping a record of all activities, examinations, and progress of the animals during the post-operative recovery period is also essential to sense issues and provide appropriate measures for the animal’s care.
Special Protocols
Specialized anesthesia techniques are used in certain research, such as those that involve cardiothoracic and neurophysiological procedures, and in certain animal groups, such as pregnant animals.
Anesthesia in Combination with NMB Agents
Neuromuscular blocking (NMB) drugs are usually used to aid mechanical ventilation by blocking spontaneous respiratory movements and also to allow suitable conditions for surgery. Common clinical use NMB drugs are classified as depolarizing or non-depolarizing agents (Bowman, 2006).
Depolarizing agents bind to muscle receptors and trigger muscle contraction producing a persistent depolarization thereby preventing muscle contractions. These drugs produce fasciculations in animals before inducing complete skeletal muscle paralysis. On the other hand, non-depolarizing agents compete with acetylcholine for receptor sites at the neuromuscular junction to produce paralysis. The effects of these drugs can be reversed by increasing the local concentration of acetylcholine using drugs such as neostigmine. Alternatively, sugammadex can be used in the case of steroid NMB agents to prevent them from acting on the neuromuscular junction (de Boer et al., 2006; Booij et al., 2009).
NMB agents are challenging to use and only produce paralysis making careful monitoring of anesthetic depth critical for humane treatment of the animals. Further, using NMB agents require the use and understanding of mechanical ventilators to control ventilation.
Mechanical Ventilators
Controlled ventilation is often required when using NMB drugs and during long-term anesthesia. As opposed to manual ventilation, mechanical ventilators allow precise control of the volume of gas delivered to the lungs, the duration of inspiration and expiration, and the pressure reached in the airway during inspiration. Ventilators achieve controlled ventilation by the application of intermittent positive pressure to the airway, which can be accomplished by directly delivering gas to the anesthetic breathing system or indirectly by compressing a rebreathing bag or bellows.
Controlling ventilation relies on the use of an endotracheal tube or the placement of a tracheal cannula if the animal is not intended to recover. These methods prove successful in comparison to using a face mask, which risks the inflation of the stomach, and laryngeal masks.
The first step of ventilating an animal involves the calculation of the required tidal volume (approximately 7 to 10 ml/kg body weight) and the selection of the respiratory rate. The following table shows suggested ventilation rates for laboratory animals.
In general, the rate is selected slightly lower than the normal resting rate of the animal when it’s conscious. In case the ventilator does not have direct settings for tidal volume and respiratory rate then they are most likely to have settings for inspiratory time and inspiratory flow rate. The calculations can be based on the following equations,
Further, some ventilators may only provide controls for inspiratory time and inspiratory: expiratory (I: E) ratio. The ratio is usually set to 1:2, however, a ratio of 1:3 and 1:4 can also be used without causing significant cardiac depression while maintaining inflation pressures below 20 cm water. Apart from these variables, a maximum inspiratory pressure should also be set on the ventilator at less than 15 cm water for small animals and not more than 25 cm water for large animals, under most circumstances.
Anesthesia of Pregnant Animals
Anesthetization of pregnant animals should be performed with great care and consideration of the adverse effects of the anesthesia on the mother and the fetus(es). In this group of animals, using good anesthetic practices such as providing oxygen before anesthesia induction, measures to reduce stress, and proper positioning of the animals, become very critical. Especially during the last third of the pregnancy, animals experience an increase in abdominal pressure which can interfere with respiratory movements and venous return.
Most anesthetics used in common practice cross the placenta which can be both advantageous and disadvantageous. Since fetuses are extremely sensitive to changes, the agents used can lead to serious acute and/or long-term effects. The residual effects of the drugs in Cesarean operation delivered fetus can cause sedation and depression in respiratory and cardiovascular systems. In this situation, naloxone (if opioid analgesic is used on the mother) and doxapram can be administered to reverse respiratory depression or stimulate respiration, respectively.
In investigations and research that require surgical procedures on the fetus, adequate anesthetization must be ensured. It is advisable to take expert advice with respect to the development stage of the fetus so that appropriate measures can be undertaken to manage responses to painful and noxious stimuli before the commencement of any procedures. The easiest approach to anesthetization is to induce deep general anesthesia in the mother using volatile agents such as isoflurane. However, the most widely used approach is to infiltrate the surgical site on the fetus with local anesthesia. Regardless of the method, the limitations and effects of each method must be considered.
Anesthesia of Neonates
Neonatal animals are a sensitive group that has increased vulnerability to hypothermia, usually have low reserves of energy, and may have poor pulmonary and circulatory function. Some species’ neonates also have a reduced capacity to detoxify a wide range of anesthetic agents. Generally, responses of neonates to anesthesia agents are significantly different from adult animals, and fasting them can lead to rapid depletion of hepatic glycogen stores resulting in hypoglycemia.
The preferable method of anesthetization of neonatal animals is by using inhalation anesthetics. The construction of the delivery apparatus can be achieved using a plastic syringe barrel. During anesthetization, appropriate care to maintain body temperature, good ventilation, and fluid balance must be taken. In large species, such as dogs, sheep, and pigs, intravenous infusion via umbilical vessels may prove more convenient. Anesthetization using hypothermia is also possible, although this approach is not recommended since its efficacy is not yet fully understood.
| Species | Breaths (min) |
|---|---|
| Pig, dog (<20 kg) | 15-25 |
| Pig (>20 kg), sheep (>20 kg), dog (>20 kg) | 10-15 |
| Primates (>5 kg) | 20-30 |
| Marmosets | 40-50 |
| Cat and rabbit (1 to 5 kg) | 25-50 |
| Guinea pig | 50-80 |
| Rat | 60-100 |
| Other small rodents | 80-100 |
Summary
- Anesthesia is defined as a temporary state of insensibility or loss of consciousness.
- The requirements of the research procedures, species, age, and background of the animal being used determine the method and agent used to induce anesthesia.
- In clinical practices, general anesthesia is preferred over local anesthesia since it results in loss of consciousness, analgesia, suppression of reflex activity and muscle relaxation.
- Anesthesia can be induced using inhalant or injectable agents.
- Anesthesia machine comprises a gas compressor, a regulator, a flow meter, a vaporizer, an anesthetic breathing system, and waste gas scavenging system.
- Good anesthesia practices involve appropriate anesthesia management and monitoring techniques, and recovery care.
- Neuromuscular blocking agents only produce paralysis. Thus efforts to monitor the depth of anesthesia is critical for humane treatment of the animals.
- Anesthetization of pregnant animals can affect their fetuses. Anesthetics can result in serious acute and/or long-term effects on the fetus. The residual effects of the drugs in cesarean operation delivered fetus can cause sedation and depression of respiratory and cardiovascular systems.
- Anesthetization of neonatal animals should take into consideration their increased vulnerability to hypothermia, usually low reserves of energy, possibly poor pulmonary and circulatory function, and reduced capacity to detoxify a wide range of anesthetic agents (certain species).
References
- Bain, J. A., & Spoerel, W. E. (1972). A streamlined anaesthetic system. Canadian Anaesthetists’ Society Journal, 19(4), 426–435.
- Bednarski, R. M., & Muir, W. (2011). Capnography in veterinary medicine. In J. S. Gravenstien, M. B. Jaffe, N. Gravenstein & D. A. Paulus (Eds.), Capnography (pp. 272–280). Cambridge: Cambridge University Press.
- Brodbelt, D. C., Blissitt, K. J., Hammond, R. A., & Neath, P. J., et al. (2008). The risk of death: The confidential enquiry into perioperative small animal fatalities. Veterinary Anaesthesia and Analgesia, 35(5), 365–373.
- Carli, F., & Asenjo, J. F. (2003). Is multimodal analgesia necessary to facilitate postoperative recovery? Techniques in Regional Anesthesia and Pain Management, 7(3), 133–139.
- Chan, E. D., Chan, M. M., & Chan, M. M. (2013). Pulse oximetry: Understanding its basic principles facilitates appreciation of its limitations. Respiratory Medicine, 107(6), 789–799.
- Cookson, J. H., & Mills, F. J. (1983). Continuous infusion anaesthesia in baboons with alphaxolone-alphadolone. Laboratory Animals, 17(3), 196–197.
- Dadd GH. The Modern Horse Doctor. Boston: JP Jewett, 1854.
- Flecknell, P. A., Hooper, T. L., Fetherstone, G., & Locke, T. J., et al. (1989). Long-term anaesthesia with alfentanil and midazolam for lung transplantation in the dog. Laboratory Animals, 23(3), 278–284.
- Fresno, L., Andaluz, A., Moll, X., & García, F. (2008). The effects on maternal and fetal cardiovascular and acid-base variables after the administration of etomidate in the pregnant ewe. The Veterinary Journal, 177(1), 94–103.
- Green, C. J., Halsey, M. J., Precious, S., & Wardley-Smith, B. (1978). Alphaxolone-alphadolone anaesthesia in laboratory animals. Laboratory Animals, 12(2), 85–89.
- Gumbleton, M., Nicholls, P. J., & Taylor, G. (1990). Differential influence of laboratory anaesthetic regimens upon renal and hepatosplanchnic haemodynamics in the rat. The Journal of Pharmacy and Pharmacology, 42(10), 693–697.
- Hunter, S. C., Glen, J. B., & Butcher, C. J. (1984). A modified anaesthetic vapour extraction system. Laboratory Animals, 18(1), 42–44.
- Jacobsen, K. R., Kalliokoski, O., Teilmann, A. C., & Hau, J., et al. (2012). Postsurgical food and water consumption, fecal corticosterone metabolites, and behavior assessment as noninvasive measures of pain in vasectomized BALB/c mice. Journal of the American Association for Laboratory Animal Science, 51(1), 69–75.
- Johnson, K. B. (2012). New horizons in sedative hypnotic drug development: Fast, clean, and soft. Anesthesia & Analgesia, 115(2), 220–222.
- Kissin, I., Motomura, S., Aultman, D. F., & Reves, J. G. (1983). Inotropic and anesthetic potencies of etomidate and thiopental in dogs. Anesthesia & Analgesia, 62(11), 961.
- Liles, J. H., & Flecknell, P. A. (1993). The effects of surgical stimulus on the rat and the influence of analgesic treatment. British Veterinary Journal, 149(6), 515–525.
- M.Glowaski, M., & A.Wetmore, L. (1999). Propofol: Application in veterinary sedation and anesthesia. Clinical techniques in small animal practice, 14(1), 1-9.
- Mather, L. E. (1983). Pharmacokinetic and pharmacodynamic factors influencing the choice, dose and route of administration of opiates for acute pain. Clinics in Anaesthesiology, 1, 17–40.
- Moll, X., Santos, L., García, F., & Andaluz, A. (2013). The effects on cardio-respiratory and acid-base variables of a constant rate infusion of alfaxalone-HPCD in sheep. Veterinary Journal, 196(2), 209–212.
- Musk, G. C., Pang, D. S. J., Beths, T., & Flaherty, D. A. (2005). Target-controlled infusion of propofol in dogs—evaluation of four targets for induction of anaesthesia. Veterinary Record, 157(24), 766–770.
- Nagel, M. L., Muir, W. W., & Nguyen, K. (1979). Comparison of the cardiopulmonary effects of etomidate and thiamylal in dogs. American Journal of Veterinary Research, 40(2), 193–196.
- Oostrom, H., Krauss, M. W., & Sap, R. (2013). A comparison between the v‐gel supraglottic airway device and the cuffed endotracheal tube for airway management in spontaneously breathing cats during isoflurane anaesthesia. Veterinary Anaesthesia and Analgesia, 40(3), 265–271.
- Rembert, M. S., Smith, J. A., & Hosgood, G. (2004). A comparison of a forced-air warming system to traditional thermal support for rodent microenvironments. Laboratory Animals, 38(1), 55–63.
- Ruane-O’Hora, T., Hall, W. J., & Markos, F. (2009). The effect of ketamine and saffan on the beta-endorphin and ACTH response to hemorrhage in the minipig. Physiological Research/Academia Scientiarum Bohemoslovaca, 58(6), 799–805.
- Schwenke, D. O., & Cragg, P. A. (2004). Comparison of the depressive effects of four anesthetic regimens on ventilatory and cardiovascular variables in the guinea pig. Comparative Medicine, 54(1), 77–85.
- Short CE. The management of animal pain: where have we been, where are we now, and where are we going? Vet J 2003; 165: 101–103.
- Sneyd, J. R., & Rigby-Jones, A. E. (2010). New drugs and technologies, intravenous anaesthesia is on the move (again). British Journal of Anaesthesia, 105(3), aeq190–aeq254.
- Stevenson DE. The evolution of veterinary anesthesia. Br Vet J 1963; 119: 477.
- Subrahmanyam M, Mohan S (2013). Safety Features in Anaesthesia Machine. Indian Journal of Anaesthesia. 57 (5): 472–480.
- Tranquilli, W. J., & Grimm, K. A. (2015). Introduction: Use, Definitions, History, Concepts, Classification, and Considerations for Anesthesia and Analgesia. Veterinary Anesthesia and Analgesia.
- Uzun, M., Kiraz, H. A., Ovali, M. A., & Sahin, H., et al. (2015). The investigation of airway management capacity of v-gel and cobra-PLA in anaesthetised rabbits. Acta Cirurgica Brasileira, 30(1), 80–86.
- Vijn, P. C., & Sneyd, J. R. (1998). I.v. anaesthesia and EEG burst suppression in rats: Bolus injections and closed-loop infusions. British Journal of Anaesthesia, 81(3), 415–421.
- Wagner, J. G. (1974). A safe method for rapidly achieving plasma concentration plateaus. Clinical Pharmacology and Therapeutics, 16(4), 691–700.
- Whelan, G., James, M. F., Samson, N. A., & Wood, N. I. (1999). Anaesthesia of the common marmoset (Callithrix jacchus) using continuous intravenous infusion of alphaxalone/alphadalone. Laboratory Animals, 33(1), 24–29.
- Winters WD, Ferrer AT, Guzman-Flores C. The cataleptic state induced by ketamine: a review of the neuropharmacology of anesthesia. Neuropharmacology 1972; 11: 303–315.
- Gurudatt C (2013). The basic anaesthesia machine. Indian J Anaesth. 57(5):438-45. doi: 10.4103/0019-5049.120138.
- Ayre, P. (1937). Anæsthesia for hare‐lip and cleft palate operations on babies. British Journal of Surgery, 25(97), 131–132
- Fish, R. E. (2008). Anesthesia and analgesia in laboratory animals. Amsterdam: Elsevier.
- Flecknel, P. (2009). Laboratory Animal Anaesthesia. Elsevier.
- Hunter, S. C., Glen, J. B., & Butcher, C. J. (1984). A modified anaesthetic vapour extraction system. Laboratory Animals, 18(1), 42–44
- Robert A. Miller (1941). “A new laryngoscope”. Anesthesiology. 2 (3): 317–20. doi:10.1097/00000542-194105000-00008.
- Robert Reynolds Macintosh (1943). “A new laryngoscope”. The Lancet. 1 (6): 205. doi:10.1016/S0140-6736(02)95524-8.
- Ramos-Cabrer, P., Weber, R., Wiedermann, D., & Hoehn, M. (2005). Continuous noninvasive monitoring of transcutaneous blood gases for a stable and persistent BOLD contrast in fMRI studies in the rat. NMR in Biomedicine, 18(7), 440–446.
- Sahbaie, P., Modanlou, S., Madanlou, S., & Gharagozlou, P., et al. (2006). Transcutaneous blood gas CO2 monitoring of induced ventilatory depression in mice. Anesthesia & Analgesia, 103(3), 620–625.
- Barter, L. S., & Hopper, K. (2011). Transcutaneous monitor approximates PaCO2 but not PaO2 in anesthetized rabbits. Veterinary Anaesthesia and Analgesia, 38(6), 568–575.
- Rembert, M. S., Smith, J. A., & Hosgood, G. (2004). A comparison of a forced-air warming system to traditional thermal support for rodent microenvironments. Laboratory Animals, 38(1), 55–63.
- O’Keeffe, N. J., & Healy, T. E. (1999). The role of new anesthetic agents. Pharmacology & Therapeutics, 84(3), 233–248
- Koblin, D. D. (1992). Characteristics and implications of desflurane etabolism and toxicity. Anesthesia & Analgesia, 75(Suppl. 4), S10–S16.
- Wood, M., & Woad, A. J. J. (1984). Contrasting effects of halothane, isoflurane, and enflurane on in vivo drug metabolism in the rat. Anesthesia & Analgesia, 63(8), 709.
- Mazze, R. I., Rice, S. A., & Baden, J. M. (1985). Halothane, isoflurane, and enflurane MAC in
- pregnant and nonpregnant female and male mice and rats. Anesthesiology, 62(3), 339–341.
- Murray, W. J., & Fleming, P. J. (1972). Defluorination of methoxyflurane during anesthesia: Comparison of man with other species. Anesthesiology, 37(6), 620–625.
- Child, K. J., Currie, J. P., Davis, B., & Dodds, M. G., et al. (1971). The pharmacological properties in animals of CT1341—A new steroid anaesthertic agent. British Journal of Anaesthesia, 43(1), 2–13.
- Box, P. G., & Ellis, K. R. (1973). Use of CT1341 anaesthetic (‘Saffan’) in monkeys. Laboratory Animals, 7(2), 161–170
- Morgan, C. J. A., & Curran, H. V. (2012). Ketamine use: A review. Addiction, 107(1), 27–38.
- Douglas, B. G., & Dagirmanjian, R. (1975). The effects of magnesium deficiency on ketamine
- sleeping times in the rat. British Journal of Anaesthesia., 47(3), 336–340.
- Dodam, J. R., Kruse-Elliott, K. T., & Aucoin, D. P. (1990). Duration of etomidate-induced adrenocortical suppression during surgery in dogs. American Journal of Veterinary Research, 51786–51788.
- Papaioannou, V. E., & Fox, J. G. (1993). Efficacy of tribromoethanol anesthesia in mice. Laboratory Animal Science, 43(2), 189–192
- Field, K. J., White, W. J., & Lang, C. M. (1993). Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Laboratory Animals, 27(3), 258–269
- Fleischman, R. W., McCracken, D., & Forbes, W. (1977). Adynamic ileus in the rat induced by chloral hydrate. Laboratory Animal Science, 27(2), 238–243
- Field, K. J., & Lang, C. M. (1988). Hazards of urethane (ethyl carbamate): A review of the literature. Laboratory Animals, 22(3), 255–262
- Severs, W. B., Keil, L. C., Klase, P. A., & Deen, K. C. (1981). Urethane anesthesia in rats. Pharmacology, 22(4), 209–226.
- Obernier, J. A., & Baldwin, R. L. (2006). Establishing an appropriate period of acclimatization following transportation of laboratory animals. ILAR Journal/National Research Council, Institute of Laboratory Animal Resources, 47(4), 364–369
- Antognini, J. F., Barter, L., & Carstens, E. (2005). Overview movement as an index of anesthetic depth in humans and experimental animals. Comparative Medicine, 55(5), 413–418.
- Bowman, W. C. (2006). Neuromuscular block. British Journal of Pharmacology, 147 (Suppl. 1), S277–S286
- de Boer, H. D., van Egmond, J., van de Pol, F., & Bom, A., et al. (2006). Reversal of profound rocuronium neuromuscular blockade by sugammadex in anesthetized rhesus monkeys. Anesthesiology, 104(4), 718–723
- Booij, L. H. D. J., van Egmond, J., Driessen, J. J., & de Boer, H. D. (2009). In vivo animal studies with sugammadex. Anaesthesia, 64(Suppl. 1), 38–44
- Thaete, L. G., Levin, S. I., & Dudley, A. T. (2013). Impact of anaesthetics and analgesics on fetal growth in the mouse. Laboratory Animals, 47(3), 175–183.
Let's work together!
Have questions? Ask anything!