$693.00
Brain Microinjection Surgery Instrument for Mice and Rats surgical covers surgical scissors, surgical forceps, orthopedic instruments, scalpels and blades, vascular forceps, needle forceps, with different models. Surgical kits also available for mice, rats, mice, cats, rabbits, guinea pigs, and hamsters.

RWD is a global leader in the design and manufacturing of advanced research and laboratory equipment. Specializing in high-precision instruments for neuroscience, behavioral science, and pharmacology.


bool(false)
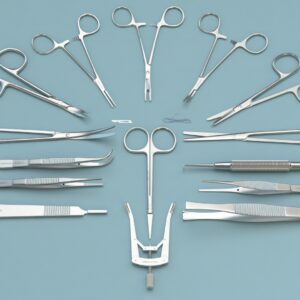
Brain Microinjection Surgery Instrument Kit for Mice
Model No. | Product Description | Qty |
|---|---|---|
S31011- 01 | Scapel Handels with Ruler 3# solid-12.5cm | 1 |
S32003-12 | 11# Scapel Blades (Box of 100pcs) | 1 |
S12005-10 | IRIS-Fine scissors (Round Type)- S/S Str/23*5mm/10.5cm | 1 |
S12004-09 | IRIS-Fine scissors (Round Type)- S/S Str/24*46mm/9.5cm | 1 |
S14014-10 | Operating Scissors (Round Type)-S/S Str/32.7*8.45mm/10.5cm | 1 |
F12005-10 | IRIS Dissecting Forceps-Str, Tip width 1mm, teeth length 14mm, 10cm | 2 |
F12006-10 | IRIS Dissecting Forceps-Light Cvd, Tip width 1mm, teeth lenght 13.5mm, 10cm | 2 |
F22002-10 | HARTMAN Mosquito Forceps-Str/20*0.8mm/10.5cm | 2 |
F31047 -12 | OLSEN-HEGAR Needle Holders with Scissors-Str, 10*2.15mm/12cm | 1 |
F35204-70 | HARTMAN Mosquito Forceps-Str/20*0.8mm/10.5cm | 0.2 |
F22003-10 | HARTMAN Mosquito Forceps-Cvd/20.8*1mm/10cm | 1 |
R51003-11 | Dual-end Micro Spatula w/tip W. 2mm x Thk. 0.3mm - Cvd/11cm | 1 |


Brain Microinjection Surgery Instrument Kit for Rat
Model No. | Product Description | Qty |
|---|---|---|
S31011- 01 | Scapel Handels with Ruler 3# solid-12.5cm | 1 |
S32003-12 | 11# Scapel Blades (Box of 100pcs) | 1 |
S12005-10 | IRIS-Fine scissors (Round Type)- S/S Str/23*5mm/10.5cm | 1 |
S12006-10 | IRIS-Fine scissors (Round Type)- S/S Str/22*45mm/10.5cm | 1 |
F12010-10 | Dressing Forceps-Str, Tip width 1.8mm, teeth length 15mm,10.5cm | 2 |
F12011-10 | Dressing Forceps-Cvd, Tip width 1.9mm, teeth length 15mm, 10cm | 2 |
F21001-12 | HALSTED Artery Forceps-Str/23.5*1mm/12.5cm | 2 |
F21002-12 | HALSTED Artery Forceps-Cvd/24*1mm/12.5cm | 2 |
F31047 -12 | OLSEN-HEGAR Needle Holders with Scissors-Str, 10*2.15mm/12cm | 1 |
S14014-12 | Operating Scissors (Round Type)-S/S Str/37.6*8.5mm/12.5cm | 1 |
F35204-70 | HARTMAN Mosquito Forceps-Cvd/20.8*1mm/10.5cm | 0.2 |
R22009-01 | ALM 4x4 Teeth Retractors-Blunt, 65mm Spread, 7.5cm | 1 |
R51003-11 | Dual-end Micro Spatula w/tip W. 2mm x Thk. 0.3mm - Cvd/11cm | 1 |
Stereotactic neurosurgery is the surgical technique used in pre-clinical research to engraft a needle or electrode at a pre-defined location in the rodent’s brain. Stereotactic neurosurgery employs advanced imaging techniques and surgical tools to translate rodent brain-related research into human applications.
In 1887, Sir Victor Horsley, while studying the connections of the cerebellum, realized that a specific method to allow precise lesioning of the cerebral nuclei is essential. This realization led to the introduction of stereotaxy in neuroscience research. Stereotactic neurosurgery has enabled the localization of targets of interest with a multifaceted set of techniques that can assess anatomic, metabolic, and functional aspects and allow precise interventions in the brain or spinal cord regions (Barbara, Damien, & Catherine, 2014).
Horsley and Clarke pioneered the principles of stereotaxic surgery. Stereotaxy required radiological tools to visualize the cranial vault and its contents before the development of magnetic resonance imaging (MRI). The stereotactic technique was later modified with the introduction of MRI to visualize and assess the tumoral pathologies. Over the last two decades, stereotaxic surgery has well established itself in the research. The stereotaxic surgery is widely applied for the symptomatic treatment of motor disorders, assessment of tumoral pathologies, interstitial radiotherapies, treatment of hydrocephaly and epilepsy, and evaluation of cerebral arteriovenous malformations. The stereotaxic methodologies integrated with computed imaging techniques have made stereotaxic surgery an important technique in neurosurgery.
Stereotactic neurosurgery is the surgical technique used in pre-clinical research to engraft a needle or electrode at a pre-defined location in the rodent’s brain. Stereotactic neurosurgery employs advanced imaging techniques and surgical tools to translate rodent brain-related research into human applications.
In 1887, Sir Victor Horsley, while studying the connections of the cerebellum, realized that a specific method to allow precise lesioning of the cerebral nuclei is essential. This realization led to the introduction of stereotaxy in neuroscience research. Stereotactic neurosurgery has enabled the localization of targets of interest with a multifaceted set of techniques that can assess anatomic, metabolic, and functional aspects and allow precise interventions in the brain or spinal cord regions (Barbara, Damien, & Catherine, 2014).
Horsley and Clarke pioneered the principles of stereotaxic surgery. Stereotaxy required radiological tools to visualize the cranial vault and its contents before the development of magnetic resonance imaging (MRI). The stereotactic technique was later modified with the introduction of MRI to visualize and assess the tumoral pathologies. Over the last two decades, stereotaxic surgery has well established itself in the research. The stereotaxic surgery is widely applied for the symptomatic treatment of motor disorders, assessment of tumoral pathologies, interstitial radiotherapies, treatment of hydrocephaly and epilepsy, and evaluation of cerebral arteriovenous malformations. The stereotaxic methodologies integrated with computed imaging techniques have made stereotaxic surgery an important technique in neurosurgery.
Before beginning the surgical procedure, it is essential to check that the animals are in good health by analyzing their appearance and general behavior. The subjects can be examined clinically to detect certain anomalies. If any abnormality is found, it is necessary to not use that animal as a subject in a neurosurgical procedure because the abnormality may cause anatomical or behavioral alterations and could interfere with the experimental results. The investigator should not use the animal if it shows reduced appetite, weight loss, abnormal exploratory behavior, bite marks, scratches, self-mutilation, prostration, hyper-responsiveness, patchy, dull, and/or ruffled fur, and abnormal posture or facial expression. Assess the animal for its health and activeness.
Anesthesia Induction
Before beginning the surgical procedure, give the animal a dose of an analgesic. After 15 minutes of the analgesia induction, induce anaesthesia and give a a subcutaneous injection of local anesthetic agent at the sites of the incision and the pressure points of the stereotaxic apparatus.
Shearing
Shearing is performed to eliminate hair and facilitate the disinfection of the skin. Do not injure the skin while shearing, since any inflammation can potentiate local superinfection and weaken the tissues of interest. Shearing should be performed in the preparation zone after the animal is sedated or anesthetized.
Setting Up the Animal in the Stereotaxic Instrument
After achieving adequate anesthesia place the animal in the stereotaxic apparatus. Make sure that all the surgical instruments are ready and available nearby the surgical area.
First Ear Bar Placement
Fix the bars in the instrument carefully. Gently grasp the head of the animal by the sides of the instrument to direct the ear canal towards the fixed bar. Carefully maneuver the head horizontally onto the bar and position the tip of the bar behind the aural bone spur. A soft characteristic click will confirm the accurate placement, do not confuse it with a louder snap that indicates the eardrum rupture if the bar is inserted deeply.
Note: Do not place the finger around the neck to hold the head of the animal, since it could block the trachea. Make sure that the animal is adequately anesthetized and appropriately positioned as poorly maintained head position can cause asphyxiation.
Second Ear Bar Placement
Maintain the head of the animal in a horizontal position to place the second bar into the second ear canal. The head of the animal cannot move from side to side if the ear bars are placed properly. Make sure that the movements are possible only at Antero- and retro-flexion points.
Tooth Bar Placement
Align the head with the centerline of the stereotaxic frame after the ear bars are properly placed. After the head alignment, fix the upper incisors of the subject on the tooth bar. For this, move the bar towards the animal, open the mouth, and slide the tooth bar behind the upper incisors. Gently pull the head of the animal forward and move the bar behind the teeth.
Nose bar Fixation
To lock the snout in position, lower the nose bar. Firmly fix the nose bar with the setscrew, but do not apply excessive pressure to ensure that the head is immobilized without injuring the animal. The head cannot move after the incisor bar and nose bar are appropriately placed.
Operating Zone Preparation
Before disinfecting the surgical site, put a rectal temperature probe in place. To minimize trauma and discomfort apply Vaseline. After the ear bars are placement under sterile conditions, disinfect the skin where the incision will be made by washing with a sterile gauze (soaked with alcohol, antiseptic soap, and solution). Discard the gauze pad if it touches the edge of the shaved zone. Antiseptics are used to disinfect the skin and kill the local flora. The antiseptic spectrum varies depending on the product and the quality of its application.
Draping
Cover the entire animal with the drape to prevent any contamination. Make sure that the overall procedure of draping remains coherent.
Cranial Surface Incision and Exposure
To incise the scalp, use a scalpel with a new blade. Make the incision confidently and precisely to avoid damaging the edges which can affect healing. The linear incision should begin rostrally, in front of eye level, and run caudally 0.5 cm behind the ear bar alignment line and includes all the layers of the skin.
Stereotaxic Landmarks
Identify the stereotaxic landmarks: bregma and lambda by visualizing the different suture lines of the bone plates on the skull surface. The accurate and precise determination of the two reference points will determine the quality of approaching the target structure. Bregma is the intersection point of the sagittal and frontal plate sutures at the rostral level of the animal’s skull. Lambda is the point on the midline and at the base of the triangle formed by the intersection of lambdoid sutures and the sagittal suture. Lambda is parallel with the virtual interaural line shown at the axis of the ear bars. Sometimes lambda is situated just anterior to the interaural line, but it could never be posterior to this line.
Make sure that the distance between the bregma and lambda is about 8.7 ±0.3 mm to ensure the accuracy of the lambda coordinates. The determination of coordinates is incorrect if the distance is greater than 9 mm or less than 8.4 mm.
Suturing
Use a sterile needle for suturing. Suturing is done to bring together and maintain the edges of the wound for rapid healing and recovery. Sutures minimize inflammation, infections, and accidental or spontaneous wound opening. Following is the procedure for suturing;
Place the animal on absorbent paper in a cage to prevent dust or litter from contacting the suture. Treat hypothermia until the anesthesia lasts. Maintain an appropriate temperature in the recovery room either by using an incubator, lamp, or a heating pad. Place some moistened food pellets in the cage to encourage feeding. Return the animals to the husbandry after complete awakening. Animals should be closely monitored postoperatively. Observe the animals for any discomfort or pain and treat the symptoms as needed. Change the cage bedding frequently to avoid contamination. If needed, clean the sutures with the help of a sterile cotton swab soaked with disinfectant. If the wound bleeds, apply direct pressure for a few minutes. If bleeding persists, re-anesthetize the animal to manage hemostasis. Suture opening can contaminate the wound so must be treated as a traumatic lesion. Antibiotics can be applied to foster healing. Treat the pain by injecting analgesics.
Assessment of the Effect of Stereotactic Implantation of Biodegradable 5-Fluorouracil-loaded Microspheres (Menei et al. 1996)
The stereotaxic neurosurgery was used to assess the efficacy of poly (lactic acid-co-glycolic acid) (PLAGA) microspheres for interstitial chemotherapy of brain tumors. In the study, two types of 5-Fluorouracil-loaded microspheres were stereotactically implanted in female Wistar and Sprague-Dawley rats. One type of 5-FU presented a fast release profile (FR), and the other exhibited a slow release pattern (SR). It was observed that the implantation of 5-FU-loaded PLAGA microspheres in the rat brain does not present any evident toxicity. The results suggested that the microspheres possess a longer sustained delivery period in vivo as compared to in vitro. It was concluded that intra-tumoral implantation of SR-type 5-FU-loaded microspheres significantly reduced the mortality of C6 tumor-bearing rats. The neurotoxic surgery has enabled the researchers to evaluate the in vitro efficacy of chemotherapeutic loaded microsphere in the rat brain.
Increasing the effectiveness of intracerebral injections (Mathon. et al., 2015)
The stereotaxic neurosurgery is a promising surgical technique for the assessment of the effects of intracerebral injections in adult and neonatal mice. The study was conducted to increase the effectiveness of intracerebral injections in adult mice and neonatal mice using the stereotaxy. The stereotaxic injections in adult mice performed in 20 min had >90% efficacy. While in neonatal mice, injections were performed in 5 min. The results suggested that the intracerebral injections using the stereotaxic neurosurgery present increased efficacy in adult and neonatal mice. It was concluded that the precise determination of intracerebral location increases the efficacy of intracerebral injections in small-sized animals such as mice. The technique using the stereotaxic arm presents a higher precision as compared to the freehand techniques.
Stereotaxic Neurosurgery for Intracerebral Microdialysis (Yanjun, Joanna, Li, & Hartmut, 2006)
Stereotaxy is also used for sampling of neurotransmitters such as microdialysis in rodents. Microdialysis enables the researchers to directly measure the unbound tissue concentrations and monitor the physiological and biochemical effects of drugs in vivo. The technique allows the sampling and quantification of substances in blood and tissue, including neuropeptides, neurotransmitters, enzyme concentration and activity, electrolytes, hormones, and pharmaceutical agents. The method also provides with an in-depth observation of pharmaceutical effects of various drug candidates on extracellular levels of endogenous substances.
Transplantation of Neuronal Stem Cells (Kevin. et al. 2011)
In the study, neuronal stem cells were transplanted using the stereotaxic neurosurgery in the mice infected with the neurotropic JHM strain of mouse hepatitis virus (MHV). The MHV results in a pathological condition similar to the demyelinating disease Multiple Sclerosis (MS). The results suggested that the stereotaxic transplantation of neuronal stem cells into the spinal cords of mice, affected by MHV, improved the re-myelination and clinical outcome. The study validated that the cell replacement therapies are vital to the treatment of chronic neurologic diseases and the in vivo models are essential in understanding the complex interactions between the transplanted cells and the host tissue microenvironment.
Barbara, F., Damien, G., & Catherine., V. (2014). Stereotaxic neurosurgery in laboratory rodents. Lyon: Springer.
Kevin, S. C., Jason, G. W., Lucia, M. W., Chris, S. S., & Thomas, E. L. (2011). Surgical Transplantation of Mouse Neural Stem Cells into the Spinal Cords of Mice Infected with Neurotropic Mouse Hepatitis Virus. J Vis Exp, 53, 2834.
Mathon., B., Nassar., M., Simonnet., J., Duigou., C. L., Clemenceau, S., Miles., R., & D.Fricker. (2015). Increasing the effectiveness of intracerebral injections in adult and neonatal mice: a neurosurgical point of view. Neurosci Bull, 6, 685-96.
Menei., P., Boisdron-Celle., M., Croué., A., Guy., G., & Benoit., J. P. (1996). Effect of stereotactic implantation of biodegradable 5-fluorouracil-loaded microspheres in healthy and C6 glioma-bearing rats. Neurosurgery, 39(1), 123-4.
Yanjun, L., Joanna, P., Li, Z., & Hartmut, D. (2006). Microdialysis as a tool in local pharmacodynamics. AAPS J, 8(2), E222–E235
| Weight | 4.41 lbs |
|---|---|
| Species | Mouse, Rat |
You must be logged in to post a review.
There are no questions yet. Be the first to ask a question about this product.
Reviews
There are no reviews yet.