
The use of animal models for studying traumatic brain injury (TBI) has several advantages, including low breeding costs, easy maintenance, and innovative techniques to create genetically modified strains. Rodent models provide powerful tools to screen and characterize putative therapeutic targets in TBI. These models efficiently recapitulate several clinical features of TBI such as closed head injuries (CHI), diffuse and axonal brain injuries, penetrating head injuries, and a combination of both. Animal models of traumatic brain injury are essential to clarify underlying injury dynamics and mechanisms and to assess the safety and efficacy of novel therapies before clinical trials. The use of genetically modified mice in TBI research has further stimulated the development of potential treatments. Experimental TBI models are popular in research because of their ability to isolate several injury mechanisms such as concussion, contusion, and penetration injuries. TBI modeling in rodents has revolutionized neuroscience research to enhance understanding of brain injuries as well as potential therapies.
Previously, models of TBI were setup in larger species such as dogs and cats, though recent developments have enabled experimental manipulations in rodents. Porcine models of TBI are more common and clinically relevant as the pig brain is closer to the human in terms of size and anatomy. However, the high cost of using large animals and the associated ethical concerns limit their wide adoption into experimental studies. In contrast, rodent models have emerged as the most widely used animal models in TBI research as they are readily available, cost-effective, amenable to behavioral, physiological and drug discovery evaluations, and offer a wide array of genetically modified strains.
Open head injury models
In open head injury (OHI) models, the injury impact is applied directly to the dura mater exposed by a craniotomy. These models include the fluid percussion and controlled cortical impact models.
Fluid percussion injury
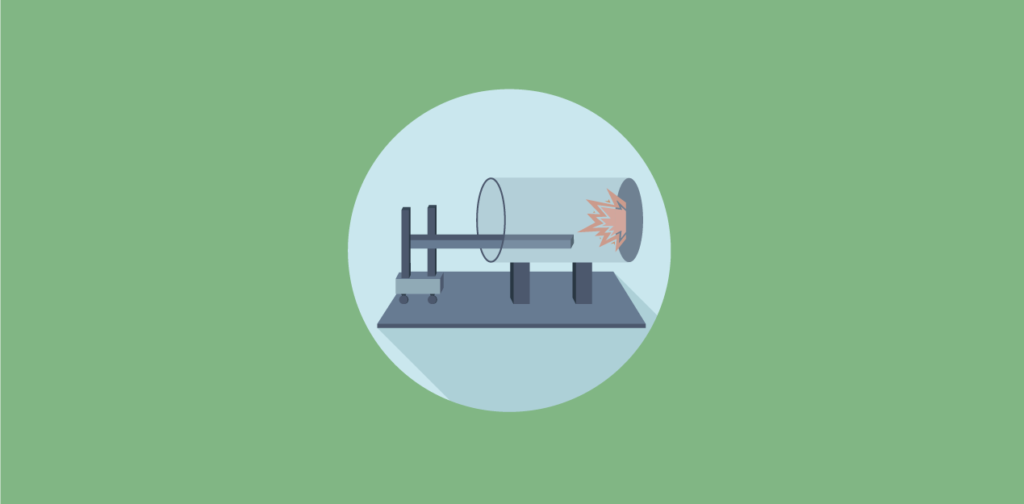
Fluid percussion injury models generate brain injury by injecting fluid onto the intact dura mater through craniotomy. Usually, the fluid is injected downwards through a midline craniotomy over the sagittal suture midway between the bregma and lambda, or sideways through lateral craniotomy over the parietal cortex. The fluid is propelled by dropping a pendulum onto the fluid reservoir. Pulse pressure, load duration, and pulse velocity are recorded. The pressure pulse of the fluid could be varied to change injury severity. FPI model could induce mixed injury including petechial and subarachnoid hemorrhage, vascular damage at the gray-white matter interface, focal necrosis, diffuse axonal injury (DAI), and cell loss.
Controlled cortical impact
Controlled cortical impact models use a pneumatic, electromechanical, or electromagnetic piston to precisely deliver tissue deformation onto an exposed dura mater. This method is used to induce primarily focal damage with extensive cortical loss, hippocampal damage, and edema, and is thereby used to mimic severe traumatic brain injury with frank tissue destruction. In these models, the impact severity is controlled by the size and design of the impactor, impact velocity, depth, and the dwell time of brain compression to produce graded injuries. CCI models do not produce ‘rebound injury’ as caused in weight-drop models.
Closed head injury models
In closed head injury (CHI) TBI models, the impact is delivered through the intact skull by directly dropping a weight on the intact skull or striking the skull with a piston, nonimpact, or inertial loading.
CHI induced by impact
In impact CHI, injury is delivered to the intact skull by dropping a weight from a pre-determined height or by a pneumatic impactor. Out of the closed head injury models, the weight-drop models are the simplest and most widely used in rats and mice for TBI research. Injury severity can be adjusted by varying height or weight. In the Marmarou weight-drop method, the animals’ head is supported on a thick foam block that allows partial head acceleration, to cause moderate to severe brain injury with diffused axonal injury (DAI). Recently, a new murine CHI model is developed that is characterized by a completely unrestrained head and body. In this model, the rodent is supported only by a sheet of aluminum foil suspended on an empty case with a thick pad at the base. The foil completely tears off during the impact delivery to allow unrestricted head and body movement of the subject as it falls into the cage. Closed head injury models are popular because of the similarity with the majority of human TBI that occur without skull fracture as compared to the FP and CCI, and simpler surgical methods that allow rapid behavioral evaluation of injury severity.
Nonimpact CHI using blast waves
Explosives and blasts could also produce brain injury through several mechanisms, including the energy of the blast wave, the acceleration and deceleration forces, and the particles that impact the head during the blast. These injuries develop with diffused edema accompanied by hyperemia and delayed vasospasm. Experimental traumatic brain injury models are developed using compressed air or gas or explosives to simulate a nonimpact blast injury.
Nonimpact CHI by inertial loading
Inertial loading CHI models use rotational forces to cause rapid acceleration of the animal’s head, followed by an extensive deceleration phase. These models are developed for larger animals, including pigs, non-human primates, and rabbits, as well as for small animals such as rats. In these models, the animal head is secured onto the mechanical system with a snout clamp. The linear motion of a piston is converted to rotational motion of the device, which leads to rotational acceleration of the head, along the coronal, sagittal, axial or oblique plane. The injury severity could be adjusted by varying the angle of rotation or pulse duration. These models have induced pathologies including subarachnoid hemorrhage, increased intracranial pressure, and a rise in serum levels, a calcium-binding protein that could be a promising biomarker for TBI. Other microscopic pathologies could also be generated such as gliosis, neuronal death, and the accumulation of phosphorylated heavy neurofilament subunits.
Controlled cortical impact
Fluid percussion injury model
Weight-drop injury model
Note: Use the midline and bregma sutures as reference points.
Concussion research (Osier. & Dixon., 2017)
Mild traumatic brain injury (mTBI) is a serious health issue that demands extensive research. Controlled cortical impact (CCI) and fluid percussion injury (FPI) are the most commonly used and well-characterized models of experimental traumatic brain injury (TBI) that have been utilized in mTBI research for the past three decades. These models could efficiently be used on several common laboratory animals, including rats, mice, ferrets, rabbits, and pigs. Also, the models could be used to produce graded injuries ranging from mild to severe. Traumatic brain injury models have been applied to study open and closed head mTBI, repeated injuries, and the long-term deficits following mTBI and concussion.
Assessing the therapeutic potential of neurotrophic factors (Sinson., Voddi., & McIntosh., 1995)
Lateral fluid-percussion brain injury (FPI) efficiently presents cognitive insufficiencies, motor dysfunction, and hippocampal cell loss in test animals. Neurotrophic factors are known to have therapeutic potential to treat neurodegenerative diseases. Also, nerve growth factor (NGF) has presented some neuroprotective behavior in models of excitotoxicity. The study was conducted to assess the neuroprotective efficacy of intracerebral NGF infusion in the FPI model of traumatic brain injury. For this, male Sprague-Dawley rats were subjected to lateral fluid percussion brain injury. NGF was introduced through a mini osmotic pump in the experimental group. NFG infusions were continued until the animal was killed at 72 hours, one week, or two weeks following the injury. Then, the animals were assessed for cognitive dysfunction and neuronal cell loss. The results show that NGF administration, in the acute, post-traumatic period following fluid-percussion brain injury, improves post-traumatic cognitive behavior in rats. Also, it was validated that the fluid percussion injury model is a powerful tool to evaluate the neuroprotective functions of neurotrophic factors.
Determination of the relation of mechanical impact and neurologic dysfunction (Hsieh. et al., 2017)
Weight-drop injury model has been used to study mechanistic details regarding traumatic brain injury. However, the relationship between the mechanical impact levels and neurological severity remained uncertain. In this study, the correlation between physical impact and graded severity was investigated at various weight-drop heights. The strike force, acceleration, and displacement during the impact were recorded. Also, the longitudinal changes in cognitive imbalances and deficits were monitored on the 1st, 4th, and 7th days following the impact. The levels of inflammatory expression markers were also measured in the frontal cortex, hippocampus, and corpus callosum using the western blot at 1 and 7 days post-lesion. It was noted that the alterations in impact pressure produced graded injuries and varying severities in the neurological score and balance function. Also, the inflammatory markers were found elevated at 1 and 7 days post-impact damage. The results implied that the severity of neurologic dysfunction and excretion of inflammatory markers are strongly correlated with the mechanical impact levels. It was concluded that the weight-drop-induced TBI model is a useful model to create graded brain injuries and induce neurobehavioral deficits.
Animal models of traumatic brain injury (TBI) are widely available and commonly deployed in neuroscience laboratories. Effective animal modeling demands careful consideration of four basic principles. First, the animal model used must be thoroughly studied and reviewed for the human disease or condition being modeled. Traumatic brain injury represents distinct clinical entities that require clear differentiation and detailed understanding of the pathophysiology, clinical course, and medical management of the subsequent conditions. Investigators using animal models must take these considerations into account to avoid misinterpretation of results. Second, the models should be selected based on the clarity of purpose regarding experimental questions and frame of reference for the investigation. Careful vigilance is required to control model artifacts having the potential to interfere with primary results. Third, the correlation between key features of the animal model and the disease being modeled is required to confirm model biofidelity. Fourth, the results observed in animals must be confirmed in clinical trials for model validation. Implementing these principles will ensure quality and accelerate clinical translation to benefit TBI research.
The animal model for traumatic brain injury should be selected carefully to meet the growing demand to understand and effectively treat TBI. The rodent models could be considered very similar to the majority of human TBI, as they closely present the pathological and behavioral outcomes following TBI. Future advances in neuroscience research and improvements in the design of various types of models will undoubtedly improve the clinical relevance and translational potential of rodent TBI models for preclinical research.
