Specifications
| Monitoring camera power |
90 mW |
| Resolution |
: 12 bits |
| Imaging speed |
: Not less than 60 fps |
| Recording mode |
: Continuous and interval recording |
| Optical zoom ratio |
: Conventional 0.67 to 4.5 times |
| Numerical aperture |
: 0.071 |
| Optional lens |
: 0.5 times shooting lens and 2 times the auxiliary lens |
| Image monitoring area |
: 14.4 x10.9 mm to 2.15 x1.62mm |
| Spatial resolution of the blood flow imaging |
: 3.3μm/pixel- 2μm/pixel |
| Effective pixels |
: 1800000 pixels /cm2 |
| Monitoring pixels |
: 656*494 pixels |
| Laser Light Source |
Source |
| Laser type |
: Laser diode |
| Wavelength |
: 785nm |
The instrument does not need any contrast agent, the time resolution can be in milliseconds, and the spatial resolution can be in microns. It also achieves the requirements of real-time observation of microvascular blood flow distribution and relative changes in values.
Laser type: laser diode, wavelengths 785nm
Monitoring camera power 90mW
Low noise 12bit fast camera for stable flow-rate measurement
Monitoring distance: 110mm
Real-time display of diameter changes and angle
Effective pixels:1800000 pixels /cm2; monitoring pixel is 656*494 pixels
Continuous recording and interval recording modes
Image monitoring area is 14.4 x10.9 mm -2.15 x1.62mm
imaging speed is not less than 60 fps in full amplitude state
ROI area and vessel diameter measurements can be added arbitrarily during the recording process or off-line analysis to support any shape and number of ROI choices
Optical magnification: conventional 0.67-4.5 times, 0.071NA, optional 0.5 times, 2 times auxiliary objective lens
increase the auxiliary objective lens. Monitoring area and blood flow imaging spatial resolution can be adjusted accordingly
The monitoring records can be exported to AVI format video files, including curves, blood flow, experimental process records
Output video can be adjusted as required
Overview
The laser speckle perfusion imager is a powerful, economical technique to image dynamic motion with high spatial and temporal resolution. It is a generally accessible vascular imaging apparatus with possible use at the bedside or during surgeries. It gives a measure of blood flow velocity by measuring the reduction in speckle contrast due to the ‘blurring’ of dynamic speckles inside a fixed camera exposure time. In addition, LSPI offers ways to infer the clinically important parameters such as blood flow and perfusion from the vessel geometry in microcirculation images.
Blood flow can be calculated by using many methods that rely on direct examination, for example, plethysmography and thermal or radio-isotope clearance strategies. These procedures are time-consuming, inconvenient, invasive, and aggravate the tissue’s regular state. Of the less invasive methods, ultrasound can be utilized. However, it is restricted to estimating higher flows at lower resolutions and is unsuccessful in estimating capillary blood flow. Strategies utilizing the Doppler effect or Laser speckle perfusion imaging technique use optical systems to record tissue perfusion, a relative measure of blood flow. These strategies have quick response times, are negligibly invasive, and are equipped for generating almost real-time, two-dimensional images of tissue microcirculation.
To date, the examination of microvascular blood flow can be executed with a few optical systems, among which laser Doppler flowmetry (LDF) and laser speckle contrast imaging (LSCI) are presently being utilized. LSPI is an innovative full-field optical strategy and a synchronized technique that does not require any scan and uses an ordinary CCD or complementary metal-oxide-semiconductor (CMOS) camera.
Principle
Laser Speckle Perfusion Imager (LSPI) depends on the following principle: the region of interest is lit up by a laser with an extended beam, and the backscattered light builds an interference pattern on the detector (a camera). Owing to the phase dissimilarity associated with the backscattered light, there are constructive and destructive interferences. The latter generates a design made out of light and dark parts on the camera. This design is known as a speckle pattern.
When tissue is illuminated with laser light from LSPI and is later imaged, the coherent laser light will create a speckle structure inside the image of the illuminated tissue. The laser speckle perfusion imager utilizes full-field illumination of the tissue and gives an instantaneous capture of image estimation points. A full-field laser perfusion imager uses a 780 nm laser light to gauge the product of normal blood speed in illuminated tissue and the concentration of moving red cells in a tissue sample volume by performing contrast examination on images procured from a video camera. It essentially measures apparent blood flow in the skin to a limited depth of around 1 mm. The velocity of blood flow is a vital parameter for understanding the physiological function and pathological alterations in microcirculation. The speckle structures inside the tissue image are an arbitrary interference pattern made by anomalies on and close to the surface of the laser-illuminated tissue.
For a stationary object, the speckle pattern shows a sharp speckle difference that stays static in time. If the object has a few individual particles experiencing movement, for example, red blood cells, then the interference pattern is dynamic and will vary in time. A spontaneous capture of a dynamic speckle design will likewise display a sharp speckle contrast, however, when a dynamic speckle structure is imaged over a limited integration period, then multiple speckle patterns become superimposed over each other, and the speckle pattern progresses toward becoming decorrelated or ‘blurred.’ The level of blurring is evaluated by the speckle contrast K that is inversely related to blood flow. The degree of decorrelation relies upon the speed and volume of blood flow inside the tissue. In LSPI, the whole tissue is illuminated with extended laser light, and a CCD camera with an imaging lens records an image containing the superimposed speckle design. The decorrelation of the speckled design over the limited integration time of the CCD apparatus is utilized to measure blood flow.
History
Laser speckle perfusion imager (LSPI) is based on the recent laser speckle contrast imaging technology which is established on the principles of speckle contrast analysis and presents an index of blood flow. The technique is employed for imaging tissue vascular structure. It has been in use since the early 1980s and as of late has been modified to utilize a charge-coupled device (CCD) camera and image processing method. It makes use of the spatial statistics of time-integrated speckle. It was first introduced by Fercher and Briers (Fercher and Briers, 1981).
Developments
Although LSPI was first presented as an alternative to Laser Doppler flowmetry for mapping microvascular perfusion in different tissues including the skin and the retina, it has now been extended to other areas and adapted to generate flow maps of the external layers of the cerebral cortex. LSPI proved to be highly suitable for the purpose, and a detailed assessment of LSPI against laser Doppler flowmetry has shown that the two methodologies convey correlating flow data and are likewise applicable and powerful, with LSPI having the benefit of a better spatial resolution. LSPI flow maps are calculated utilizing the fluctuating intensity of the arbitrary interference effect called speckle. Both methods determine flow data based on the same physical phenomenon and yield similar outcomes. Specifically, laser Doppler flowmetry and LSPI were established as equally suitable for the description of CBF changes, CO2 challenge, or after middle cerebral artery occlusion of rodents in animal models.
LSPI has been profoundly adopted for use in neuroscience, for example, blood flow imaging of neurovascular pathologies, functional revival, and even human cortical blood flow imaging throughout neurosurgery. The dynamic imaging capacity of LSPI emerges from connections between coherent photons and tissue. At the point when these photons collaborate with moving elements, the inconsistency of the distinguished intensity variations of the speckle pattern changes, which causes spatial blurring while averaging over a set exposure period. This blurring is specifically connected to the change in the intensity autocorrelation function g2(t), which is consecutively linked to sample movement through the field autocorrelation function g1(t). Nearly all speckle contrast models aim to relate variations in speckle contrast to variations in the autocorrelation decay time, etc. The autocorrelation time is said to be inversely related to the speed of the scatterers, with multiple scattering theories including a weighting term for each added dynamic scattering occurrence. Chronological intravascular scattering has been examined with regard to diffuse correlation spectroscopy; however, it still needs to be analyzed for LSPI.
Efforts to build up a more methodical, neurovascular-specific comprehension of speckle imaging depend on revisiting hypotheses utilized in dynamic light scattering models and including practical data about the complex spatial structure of the vascular system. The latest research has demonstrated that the level of intravascular multiple amid speckle imaging is lower than the photon dispersion limit and is considerably different when imaging surface vessels and parenchyma. Moreover, speckle visibility models that integrate multiple scattering as an element of vessel quality have been shown to consistently predict the association between, etc, and red blood cell (RBC) speed fluctuations in surface vessels in vivo. None of these techniques have studied the sensitivity of the LSPI signal to variations in flow in particular areas of the vascular bed. The speckle contrast signal emerges from a collective average of all dynamic scattering occurrences experienced by detected photons. Deciding on the sensitivity of speckle contrast imaging to changes in flow, thus, needs an account of intravascular scattering not just directly under the detector, but also in each vessel that a detected photon may have traveled through.
Humeau-Heurtier et al. evaluated the microvascular blood flow using the generalized differences algorithm. LSPI technique has a drawback of leading to a huge quantity of data. Efforts to perform a spatial averaging of blood flow by clinicians only lead to a reduced spatial resolution for the analyzed data. To overcome the problem of poor spatial resolution, a new post-acquisition visual representation for LSPI perfusion data by means of the generalized differences (GD) algorithm was proposed. For the experiment, LSPI produced 15 simulated images, each one 30 × 30 pixels
2. This technique produced a new single perfusion image which in itself presented the changes in the blood flow on the complete images of the stack. Furthermore, this latest image had the benefit of having a similar spatial resolution as the original images. The data confirmed that the generalized differences algorithm presented a new method of visualizing LSPI perfusion data.
Laser speckle imaging has become a pervasive technique for imaging blood flow in various tissues. However, because of its wide-field imaging characteristic, the measured speckle contrast is a depth-integrated measure and understanding of baseline values. The depth-dependent sensitivity of those values to alterations in basic flow has not been comprehensively assessed.
Davis et al. presented a newly developed procedure for measuring the autocorrelation function for ordered flow in 3D geometries. Laser speckle contrast imaging was used to determine the sensitivity of LSCI to variations in underlying velocity utilizing Monte Carlo simulations of light scattering in the cortical vasculature and a relative account of blood flow in all vessels. It was established that the regularly used type of g1(t), depends on assumptions concerning the quantity and form of scattering that are not correct. In addition, it was demonstrated that using the generally used speckle contrast models leads to almost the same sensitivity to underlying flow.
Blood flow and perfusion are essential experimental microcirculation factors. Laser speckle flowmetry has suffered from some speculation regarding the estimation of the inverse relation involving decorrelation time (τ
c) and blood flow velocity (V) i.e., 1/τ
c = αV.
Nadort et al. utilized a microcirculation imager, i.e. integrated sidestream dark field – laser speckle contrast imaging (SDF-LSCI) device. The SDF – LSCI empirically investigated the effect of the optical properties of scatterers on α in vitro and in vivo. The imaging tip of the integrated SDF-LSCI appliance was lightly set in contact with the chorioallantoic membrane tissue of the chick embryo to avoid disruption of blood flow and in vivo image frames were recorded. The apparatus was secured by hand for sublingual microcirculation imaging while it was fixed to a stand for chick embryo microcirculation imaging. The data concluded that SDF-LSCI provided a quantifiable estimate of flow velocity as well as vessel morphology, allowing the quantification of the clinically significant blood flow, velocity, and tissue perfusion.
Numerous studies have revealed that the LSCI has great potential to be an important cerebral blood flow measuring procedure for neurosurgery. Yet, the quantitative precision and sensitivity of LSCI are inadequate and vastly reliant on the exposure duration. An addition to LSCI called multi-exposure speckle imaging (MESI) has overcome these restrictions.
Richards et al. used the LSCI to evaluate patients going through brain tumor resection intraoperatively. This experimental research measured several exposure times from the same cortical tissue area and assessed images separately as single-exposure LSCI and combined using the MESI model. The results revealed that the MESI measurements presented the widespread flow sensitivity for sampling the extent of flow in the brain, narrowly followed by the shorter exposure times. In conclusion, intraoperative MESI can be conducted with high quantitative precision and sensitivity for cerebral blood flow monitoring.
LSPI has also been used to evaluate the chronic wide-field imaging of brain hemodynamics. Chronically assessing brain activity throughout active and social behavioral situations has provided significantly relevant physiological information on pathological conditions.
Miao et al. presented a new standalone micro-imager for examining the cerebral blood flow (CBF) and total hemoglobin (HbT) behavior in the freely moving status of animals utilizing the laser speckle contrast imaging (LSCI) and optical intrinsic signal (OIS) techniques. Moreover, a novel cranial window technique, utilizing contact lens and wide field optics, was presented to attain the chronic and wide-field imaging of rat’s cerebral cortex. Chronic imaging revealed enhanced CBF and HbT in the motor cortex while the rats were going up the cage wall. Additionally, after the climb, CBF completely returned to the baseline while HbT demonstrated a late recovery. The micro-imager equipment offers the new potential for brain imaging in cognitive neuroscience experiments like analysis of brain activities in social activities and social impulses.
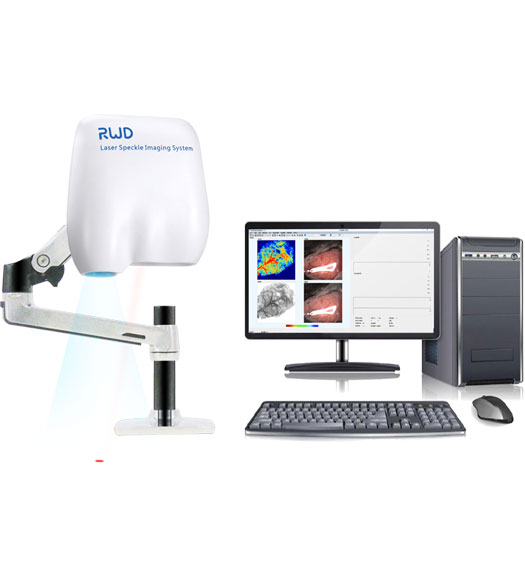
Apparatus and Equipment
RFLSI Pro Laser Speckle Perfusion Imager comprises a micro-imaging system, software system, and a laser light source. The instrument does not require a contrast agent and is able to achieve real-time dynamic blood flow monitoring and video imaging. The monitoring distance is 110mm. The system allows the arbitrary addition of regions of interest (ROI) and vessel diameter measurements during the recording process or during off-line analysis to support any shape and number of ROI choices. The monitoring area and blood flow imaging spatial resolution can be adjusted accordingly as well. The monitoring records can be exported to AVI format video files, including curves, blood flow, and experimental process records, and the output video can be adjusted as required.
Protocol
The general purpose of laser speckle perfusion imaging (LSPI) is to achieve a powerful and economical technique of imaging dynamic motion with high spatial and temporal resolution. It provides an important foundation for understanding the organization, organ pathology, and physiological markers.
The first step to accomplishing the goal of LSPI is to set up the imaging device and software. This is achieved by mounting a camera with a macro zoom lens to a vertical stage or surgical microscope. It is even possible to use an objective microscope lens or a simple two-lens system in place of the macro zoom lens keeping in mind the required magnification. Next, the camera software to control the camera should be tested to verify that the desired object is focused at the necessary height. Then, a laser diode is set up along with a collimation kit to ensure the illumination of the object with divergent laser light. Once the object is illuminated, turn off all other surrounding light to make sure that the laser light is uniformly enlightening the complete viewable area of the camera.
Typically, red laser light can be utilized because it is less demanding to exhibit how to construct the framework, yet infrared laser light can also be utilized and has the extra advantage of penetrating further into the tissue. Additionally, infrared light may even be utilized with the room lights on provided that suitable filters are used in front of the camera to obstruct visible light.
Next, the software of the camera device is utilized to obtain images and furthermore determine the values of the speckle contrast. The object of interest is placed inside the field of view of the camera, and the height of the camera is altered accordingly. The focus of the lens is also adjusted until clear-cut images of the object are obtained. It is also important to ensure that adequate laser light gets through to the imaging device but does not saturate it. The next step revolves around utilizing the histogram of the image to change the laser power and excite the majority of the camera pixels to approximately half of their capacity. Before beginning the experiment, remember to choose the number of images that are required and the amount of averaging that needs to be done. When the experiment has begun, variations in blood flow can simply be observed by choosing regions of interest or by producing images of relative blood flow.
Applications
Evaluation of blood-flow dynamics in skin flaps
Du et al. utilized full-field laser perfusion imaging (FLPI) to investigate the temporal changes in the circulation in rat dorsal delay random flaps. Adult male Sprague-Dawley rats were used to estimate the flap viability by evaluating the role of hemodynamic changes in the delay process. This was achieved by using the LSI method for real-time assessment of blood flow. Rats underwent a modified McFarlane skin flap procedure and were randomly divided into two groups; delay procedure and controls. The delay group had a bipedicle vascular delay procedure performed, wherein two longitudinal incisions were made, and the flap was completely raised, including the panniculus carnosus. In both groups, the skin flap was sutured in place after relocation using a 4-0 suture. Full-field laser perfusion imaging measures (expressed in BPU) were taken post-operatively at times 0, 1 hour and on days 1, 4, and 7. Further, the contrast images were processed to create a color-coded live flux image in which red denoted high flow speed while blue denoted low flow speed. The measures were taken at three regions of the skin flap; proximal, middle, and distal. Based on the data it was concluded that the delay procedure led to improved vessel diameter, flow speed, and flap viability, thereby reducing the probability of flap necrosis.
Evaluation of blood perfusion in stretched and rotated skin flaps
Nguyen et al. employed the laser speckle contrast imaging (LSCI) technique to examine blood perfusion by LSCI after stretching or rotating arbitrary pattern skin flaps in a porcine model. Skin flaps of random patterns (1 x 4 cm) were dissected from the side of eight pigs. LSCI charted an area of 24 × 24 cm, and the flaps were dissected in a region where perfusion was homogenous, i.e., without any perforators. After the flaps were dissected, a stabilization period of one hour was given before beginning the experiment. Forceps and/or a digital scale was utilized to stretch the skin flaps with a force of 3 or 10 N and/or rotated 45° or 90°. The skin flaps were allowed additional time for stabilizing after each treatment before perfusion was examined. LSCI was used to measure blood perfusion, and the data concluded that to facilitate the rectification of a defect, a settlement must be made between the length of the flap and the point to which it is stretched. Furthermore, the rotation of the skin flap apparently did not have an extensively damaging effect on perfusion.
Evaluation of the role of autophagy in chronic cerebral hypoperfusion
Zou et al. aimed to determine whether autophagy plays a role in neuronal damage and Aβ deposition in chronic cerebral hypoperfusion (CCH). The two-step bilateral common carotid artery occlusion (BCCAO) was performed to produce CCH in Sprague Dawley rats. The cerebral blood fluid (CBF) variations were monitored by means of laser speckle contrast imaging (LSCI). Prior to assessing CBF, a skin incision was performed on the rats to expose the skull. The periosteum was removed, and a 5 x 10 mm
2cranial window of the cerebral cortex was thinned until the pial vasculature was observable. A non-contact laser probe was placed around 7.5 cm above the frontal-parietal cortical region of the brain. Cognitive changes and pathological changes, including neuronal injury, white matter lesions, and β-Amyloid (Aβ) deposition were assessed by approved methods. Autophagy was examined by means of western blotting and immunohistochemistry. The data concluded that while rat CBF slowly improves after two-step BCCAO, cognitive impairment CCH becomes worse with time. Autophagy plays a vital part in the development of neuronal damage and cognitive decline, in addition to intracellular Aβ aggregation.
Evaluation of impaired neurovascular coupling responses
Tarantini et al. presented an easily adaptable and relatively fast protocol for the measurement of neurovascular coupling responses in mice in both geroscience and Alzheimer’s disease (AD) studies. Laser speckle contrast imaging (LSCI) allowed for quick and minimally invasive observation of alterations in regional Cerebro-microvascular blood perfusion. Mice were endotracheally intubated and
ventilated. The skin overlying the desired imaging area was shaved. After injecting a local anesthetic at the incision path, a 1 cm longitudinal incision alongside the midline of the skull was made. The skin was pulled sideways to expose the skull and was held in place using bulldog serrefines. The periosteum was removed using fine forceps, and the surface of the skull was cleaned. The borders of the thinned skull cranial window were marked by using a permanent marker. A precision dental drill was utilized for thinning the skull on top of the area of interest until translucent. The laser speckle contrast imager was placed above the thinned skull of a mouse and data was obtained. After each experiment, the brain should be instantly removed and hemisected for successive biochemical and histological analyses. The simulation procedure implemented to explore neurovascular coupling includes ten stimulation presentation trials. The study provided comprehensive guidelines for the successful measure of neurovascular coupling responses in anesthetized mice set up with a thin skull cranial window. The method will allow clinicians to process larger cohorts in a shorter time duration.
Evaluation of ischemic stroke induction and mesoscopic imaging assessment of blood flow
Balbi et al. developed a process for stroke induction in conscious, head-fixed mice in order to avoid possible confounding from
anesthesia. They also used laser speckle contrast imaging and wide-field calcium imaging to demonstrate the outcome of cortical spreading ischemic depolarization following a stroke in both anesthetized and awake mice over a spatial scale surrounding both hemispheres. GCaMP3 mice were utilized to examine spreading waves of activity following a stroke. The mice were anesthetized with isoflurane and then placed in a
stereotactic frame. The skin flanked by the ears and the eyes were shaved and appropriately cleaned with betadine dissolved in water and ethanol. The skin surrounding the occipital, parietal, and frontal bones was removed. Dental cement was utilized to glue a head-fixing screw to the cerebellar plate. The cement stayed transparent after it solidifies, and the region of interest easily seen through the final result. To induce a focal ischemic stroke in awake mice, the photothrombotic model was utilized which is based on the light-dependent production of reactive oxygen species. Laser speckle imaging was executed before, immediately after stroke induction, and every other day throughout the first week following the stroke in awake head-fixed mice. With a combined method, ischemic depolarizing waves circulating across the cortex 1 to 5 min following stroke induction was observed in genetically encoded calcium indicator mice.
Evaluation of hypoperfusion in hyper-early reperfusion after cerebral ischemia:
He et al. examined cerebral blood flow (CBF) in a hyper-early phase of reperfusion by using the laser speckle contrast imaging technique. Twenty-seven male Sprague-Dawley (SD) rats were utilized in this study.
Middle cerebral artery occlusion (MCAO) surgery was performed on rats with or without treadmill training followed by reperfusion. Laser speckle images of the rats were obtained prior to MCAO surgery, 30 min following the onset of surgery, and 1, 2, 3, and 24 hours post-reperfusion. The laser speckle images (696 × 512 pixels) were taken at 23 frames per second with a blood vessel and flow imaging device above the skull. 200 continuous frames of speckle images were recorded in each trial to record CBF measurements. The data concluded that exercise preconditioning, as a neuroprotective method, has the capacity to improve the after-effects of ischemia. In the hyper-early stage of reperfusion, exercise preconditioning decreased perfusion of arteries and veins, which can stimulate the intervention-induced neuroprotective hypoperfusion after the onset of reperfusion.
Evaluation of blood flow and microvascular reactivity in patients affected by Raynaud’s phenomenon
Della Rossa et al. investigated the blood flow and microvascular reactivity by laser speckle perfusion imager (LSPI) in patients affected by Raynaud’s phenomenon (RP) at baseline and after dynamic simulations. A total of 76 subjects were examined including 20 healthy subjects and 56 patients with RP. Blood flow through the skin was analyzed throughout the research utilizing a high frame rate LSPI. The wavelength of the laser was 785 nm. The laser scanning device was mounted 20 cm over the skin of the dorsum of the hand. Afterward, the post-occlusive hyperemia test and the cold test was performed on the patients. The study also tested the efficiency of LSPI in the discerning primary from secondary Raynaud’s phenomenon, with specific reference to systemic sclerosis (SSc) related to Raynaud’s phenomenon. The data shows a clear-cut variation of the dynamic of microcirculation in SSc-RP as compared to a primary form of the disease and healthy subjects.
Strengths and Limitations
Strengths
Forrester et al. compared the data from the laser speckle perfusion imager against the laser Doppler perfusion imager. Previously, laser Doppler perfusion imaging (LDI) was being utilized in multiple numbers of clinical purposes; but, LDI instruments generated images of low resolution and extensive time was utilized for scans. The research compared the measurements of human skin with measurements of surgically exposed rabbit tissue made using a laser speckle perfusion imager and a commercial laser Doppler perfusion imaging device. For this experiment, LSPI camera distance and magnification were modified so that the area of interest used the entire camera field of view and provided blood flow image resolutions of 768 × 494 pixels for every single measurement. The data revealed some advantages that the novel LSPI method had over the conventional LDI method, e.g. better blood flow parameters, higher temporal resolution of hyperaemic response, and versatility in a clinical surroundings.
Hence, the major advantage of the laser speckle perfusion imager is the instantly apparent high resolution of the images captured with the device. Not only is the device capable of portraying areas of higher and lower perfusion, but it also depicts subtle aspects of the vascular structures inside the captured image. This feature provides a clinical advantage, where the impact of occlusion and hyperemia can be examined at the vascular stage utilizing LSPI.
Another feature is that LSPI instruments create a highly contrasting image of the laser-illuminated tissue. These images are valuable for deciding the anatomical boundaries related to the perfusion areas displayed in the blood flow maps. In LSPI, the black and white image is taken as a real-time video, offered at the standard video resolution, and refreshed at video rates. Consequently, the video display can be exceptionally valuable for real-time clinical examination and for placing the device over the tissue of interest.
In fact, tissue blood flow because of vascular disruption is exceedingly dynamic and the response time of the apparatus can fundamentally influence a blood flow measurement. The quick LSPI temporal response gives it a distinct advantage over other instruments. It has the capacity to screen high-frequency blood flow variations, capturing thousands of images in the time it takes to finish one full-sized scan by a laser Doppler imager.
Additionally, the LSPI instrument utilizes a polarized source of light in combination with a crossed polarization filter in the receiving optics to limit the impacts of specular reflection amid light estimation. The utilization of crossed polarization filters is a customary technique for reducing specular reflection during optical evaluation.
The data from LSPI also appears to have incredible reproducibility. The quick acceptance of LSPI in research is most likely due to the relative simplicity and minimal expenditure to assemble the instrument, compared with different methods, for example, MRI or CT. The growth and progress of this method have been the subject of numerous researches. LSPI is presently employed in several medical fields, for example, dermatology, plastic & reconstructive surgery, cardiology, vascular solution, diabetology, neuroscience, and ophthalmology among others. In cardiovascular research, LSPI can be utilized to examine the damage to tissue blood supply incited by pathologies, for example, diabetes, Raynaud’s phenomenon, or peripheral vascular infections. Observing blood flow with LSPI can then allow an early diagnosis or an assessment of the development of such ailments.
A distinct added advantage of utilizing LSPI is that it can be efficiently combined with other imaging modalities, permitting the precise spatial and temporal correlation of optical signals. For example, relative variations in cerebral blood volume and hemoglobin saturation can be accomplished by recording inherent optical signals at determined wavelengths (i.e., green or red, respectively) synchronized with cerebral blood flow changes captured by LSPI. In addition, spectroscopic estimations utilizing different wavelengths—as opposed to a single light source of a particular, limited range—can generate quantitative information on hemoglobin saturation parallel with relative variations in cerebral blood flow by LSPI.
Furthermore, LSPI has been effectively incorporated into multi-modular imaging frameworks, which visualize membrane potential variations in the cerebral cortex, or image deviations of pH in the nervous tissue. These methodologies are profoundly relevant and influential, in light of the fact that the precise spatial and temporal match of individual modalities provides the chance to make particular inferences about their coupling designs.
Limitations
Like any other technological device, LSPI also has a few limitations. First, LSPI gives blood flow measurements in arbitrary units: no absolute measures as ml g−1• min−1 tissue is feasible. Another downside of the LSPI method is that it delivers a lot of information: the frequency sampling of the frames can be of a few Hz, and the video recordings generally span over a few minutes.
One of the major drawbacks of LSPI has been its restricted quantitative flow and perfusion certainty reflecting the genuine physiological state, shown by high deviation and a weak association with in vivo absolute flow velocities in animal research. This imprecision originates from the way that conventional LSPI frameworks image cerebral blood flow utilizing camera exposure time alone, which restricts flow sensitivity to a small range.
Likewise, single-exposure LSPI is easily affected by different instrumentation factors, including illumination varieties, noise during imaging sessions, and alterations in the amount of dynamic in opposition to static scattering contributions in the recorded light. This restricts LSPI to intra-patient utilization at a single time point and inhibits the formation of quantitative thresholds required to aid in surgical management.
Summary
- The laser speckle perfusion imager (LSPI) is a vascular imaging apparatus based on the laws of speckle contrast analysis and provides an index of blood flow.
- The LSPI uses a charge-coupled device (CCD) camera and an image processing method.
- The LSPI technique has extended to several medical fields like dermatology, plastic & reconstructive surgery, cardiology, vascular solution, diabetology, neuroscience, and ophthalmology, etc.
- LSPI has the advantage of better blood flow parameters, higher temporal resolution, and high reproducibility of data.
- LSPI has the drawback of LSPI delivering blood flow parameters in arbitrary units and generating overly large amounts of information.
References
Du, Z., Zan, T., Li, H., & Li, H. (2011).
A study of blood flow dynamics in flap delay using the full-field laser perfusion imager. Microvascular Research, 82, 284–290. doi: 10.1016/j.mvr.2011.09.010
Humeau-Heurtier, A., Mahé, G., & Abraham, P. (2015).
Microvascular blood flow monitoring with laser speckle contrast imaging using the generalized differences algorithm.Microvascular Research, 98, 54–61. doi: 10.1016/j.mvr.2014.12.003
Nguyen, C. D., Sheikh, R., Dahlstrand, U., Lindstedt, S. & Malmsjö, M. (2017
). Investigation of blood perfusion by laser speckle contrast imaging in stretched and rotated skin flaps in a porcine model. J Plast Reconstr Aesthet Surg, 71(4), 611-613. doi: 10.1016/j.bjps.2017.08.030.
Fercher, A. F., Briers, J. D. (1981). Flow visualization by means of single-exposure speckle photography. Opt Commun, 37, 326–330.
Forrester, K. R., Stewart, C., Tulip, J., Leonard, C., & Bray, R. C. (2002).
Comparison of laser speckle and laser Doppler perfusion imaging: measurement in human skin and rabbit articular tissue. Medical & Biological Engineering & Computing, 40, 687-697.
Zou, W., Song, Y., Li. Y., Du. Y., Zhang, X. & Fu, J. (2018).
The role of autophagy in the correlation between neuron damage and cognitive impairment in rat chronic cerebral hypoperfusion. Mol Neurobiol, 55, 776–791. doi: 10.1007/s12035-016-0351-z
Miao, P., Zhang, L., Li, M., Zhang, Y., Feng, S., Wang, Q., & Thakor, N. V. (2017).
Chronic wide-field imaging of brain hemodynamics in behaving animals. Biomedical Optics Express, 8(1), 436-445. doi:10.1364/BOE.8.000436
Davis, M. A., Gagnon, L., Boas, D. A., & Dunn, A. K. (2016).
Sensitivity of laser speckle contrast imaging to flow perturbations in the cortex. Biomedical Optics Express, 7(3), 759-775. doi:10.1364/BOE.7.000759
Tarantini, S., Fulop, G. A., Kiss, T., Farkas, E., Zölei-Szénási, D., Galvan, V., Toth, P., Csiszar, A., Ungvari, Z., & Yabluchanskiy, A. (2017).
Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. GeroScience, 39, 465–473. doi:10.1007/s11357-017-9980-z
Balbi, M., Vanni, M. P., Silasi, G., Sekino, Y., Bolanos, L., LeDue, J. M., & Murphy, T. H. (2017).
Targeted ischemic stroke induction and mesoscopic imaging assessment of blood flow and ischemic depolarization in awake mice. Neurophotonics, 4(3), 035001. doi: 10.1117/1.NPh.4.3.035001
Richards, L. M., Kazmi, S. M., Olin, K. E., Waldron, J. S., Fox, D. J., & Dunn, A. K. (2017).
Intraoperative multi-exposure speckle imaging of cerebral blood flow. Journal of Cerebral Blood Flow & Metabolism, 37(9), 3097–3109. doi: 10.1177/0271678X16686987
He, Z., Lu, H., Yang, X., Zhang, L., Wu, Y., Niu, W., Ding, L., Wang, G., Tong, S., & Jia, J. (2018).
Hypoperfusion induced by preconditioning treadmill training in hyper-early reperfusion after cerebral ischemia: a laser speckle imaging study. IEEE Transactions on Biomedical Engineering, 65(1), 219-223. doi: 10.1109/TBME.2017.2695229
Della Rossa, A., Cazzato, M., d’Ascani, A., Tavoni, A., Bencivelli, W., Pepe, P., Mosca, M., Baldini, C., Rossi, M., & Bombardieri, S. (2013).
Alteration of microcirculation is a hallmark of very early systemic sclerosis patients: a laser speckle contrast analysis. Clinical and Experimental Rheumatology, 31(76), S109-S114.
Nadort, A., Kalkman, K., Van Leeuwen, T. G., & Faber, D. J. (2016).
Quantitative blood flow velocity imaging using laser speckle flowmetry. Scientific Reports, 6. doi: 10.1038/srep25258