Researchers all over the world agree that precise, detailed, and accurate source documentation is crucial for any clinical trial. So let’s explore the meaning of ‘source documentation.’ Source documentation can be any medical record or form kept for each participant – prior, during, and after the clinical trial procedures (Bargaje, 2011).
Source documentation can consist of hospital records, notes, diaries, checklists, transcriptions, X-rays, charts, and much more. As mentioned above, records should include the time before, during, and after the study. Most of all, source documentation needs to be clear, precise, and complete.
Why is source documentation important?
Source documentation is the crucial link that permits investigation and validation of data at any time, by any research body. As audits are crucial, good documentation can only facilitate the process, supporting successful outcomes. Source documentation gives participants a unique chance to track the complete research journey of each participant. At the same time, good source documentation can prove that ethical rights and subjects’ well-being have been respected during research.
We should not forget that good source documentation gives researchers the exciting opportunity to reconstruct the study and add more valuable insights to the existing knowledge. Each small grain of knowledge helps researchers load the medical sack of progress.
How to ensure good source documentation practices?
As medical trials determine people’s physical and emotional well-being, many ethical issues are involved in research. Thus, professionals and institutions need to follow numerous strict regulations. Due to the wide range of regulations, authorities have taken a crucial step: they’ve imposed research standards.
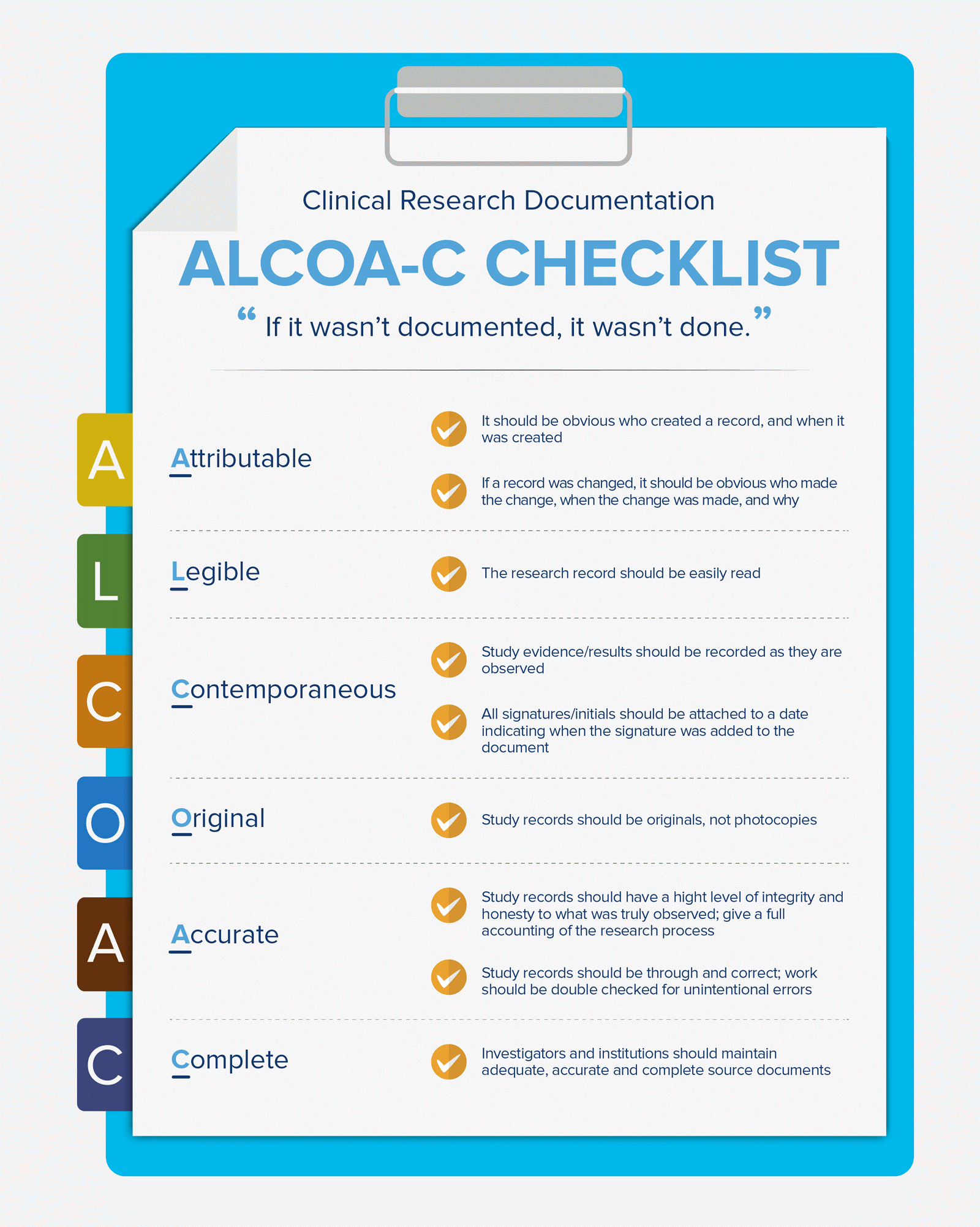
The U.S. Food and Drug Administration (FDA) became the first body to introduce standards. In a simple form, all main aspects of good documentation have been presented. This form is known as ALCOA-C (ALCOA before). ALCOA-C includes some crucial definitions and regulations of good source documentation. In addition, it tackles all possible issues that may emerge from any electronic source documentation.