
Since the discovery of chromosomes, the cytogenetic field has evolved rapidly. The techniques have unfolded several hidden mysteries of chromosomes. And later, it gave rise to two branches: molecular and clinical cytogenetics. The studies in clinical cytogenetics suggested that the structural and numerical aberrations of chromosomes (autosomal and sex) are the cause of several abnormalities in human beings. For example, Down’s syndrome, Turner’s syndrome, cancer, etc. The molecular cytogenetic techniques (for example FISH, multicolor karyotyping, CGH, etc.) proved to be an essential tool to study genetic disorders and find their possible treatments.
These techniques have also been widely used in the process of prenatal and postnatal diagnosis by researchers. Moreover, doctors also suggest pregnant women for prenatal cytogenetics analysis and prenatal ploidy analysis to detect any genetic abnormality in the fetus (if they have any family history of genetic diseases)[1]. The analysis is generally done by using the karyotyping and FISH technique.
The advanced cytogenetic techniques such as microarray aid in the high-resolution analysis and have the ability to map the copy number changes in the genome. These techniques have also proved very helpful in the diagnosis, therapy, and monitoring of the effect of the treatment on any cancer patient. We’ll discuss clinical cytogenetics and its relevance in the study of chromosomal aberrations.
Few categories[1] of chromosomal aberrations:
In Clinical Cytogenetics – Pt.1 we discussed points 1-3 above and learned various chromosomal aberrations that included deletions, inversions, substitution, translocation, and aneuploidy of the chromosomes. We also talked about the mechanisms that lead to these aberrations and the diseases they cause in human beings.
So, for this article, we will continue looking into these categories, particularly categories 4-7, as follows;
Infertility is the inability to conceive a child by a couple, despite having unprotected intercourse for at least one year. Both male and female factors can be responsible for infertility. The causes of infertility in both the sexes include:[1]
| S.No. | Causes of female infertility | Causes of male infertility |
| 1. | Fallopian tube blockage or adhesion | Varicocele (swelling of veins in the testes) |
| 2. | Cervical and uterine abnormality | Hormone imbalances |
| 3. | Endometriosis (endometrial tissue grows outside of the uterus) | Ejaculation issue ( premature ejaculation, genetic disease, blockage of testicles, etc) |
| 4. | Early menopause | Oligospermia (low sperm count) |
| 5. | Ovulation disorders | Chromosome defects and Cancer |
Sometimes, amenorrhea is the cause of female infertility. It is of two types: (a) primary amenorrhea, which is the absence of menstruation in females; and (b) secondary amenorrhea, the occurrence of discontinuous menstruation. Cytogenetics studies have found primary amenorrhea as the major cause of infertility in women.
The key event in the history of clinical cytogenetics was when a scientist, James, developed a technique to determine the sex of the fetus from the amniotic fluid by the Papanicolaou method using Giemsa stains. Now, three procedures are available to collect amniotic fluid for the analysis and these are; Amniocentesis, Chorionic Villus Sampling, and Percutaneous Umbilical Cord Sampling (PUBS).
The collection of amniotic fluid by transabdominal and transcervical puncturing of the uterus is called amniocentesis. This technique was developed and has been practiced since the 1930s[1] and today it’s a common tool to detect prenatal and postnatal cytogenetic and molecular abnormalities.
In the Chorionic villus sampling technique, developing placental cells are taken from pregnant women through the abdomen or cervix. And, PUBS (also called Cordocentesis) involves the collection of blood through the abdomen from a vein of the umbilical cord of pregnant women. These procedures are very helpful to detect any genetic abnormality in the fetus and to provide genetic counseling. Table [1] below will provide a brief of all three techniques.
| Techniques | Time period recommended for testing (weeks) | Diagnostic/cytogenetic analysis |
| Amniocentesis | 16-18 | ● Karyotype ● Biochemical studies |
| Chorionic Villus Sampling | 8-11 | ● Karyotype ● Cytogenetic analysis ● Enzymatic studies |
| Percutaneous Umbilical Cord Sampling (PUBS) | 18-23 | ● Karyotype and DNA analysis ● Detection of disorders and infections. |
(a) Amniocentesis (b) Chorionic Villus Sampling (c) Percutaneous Umbilical Cord Sampling (PUBS)
Figure: Image showing three procedures/techniques for prenatal cytogenetic analysis. Source: https://www.pregnancyhealth.net/chorionic-villus-sampling-cvs-procedure-risks/ , http://pennmedicine.adam.com/content.aspx?productid=14&pid=14&gid=000229 |
There are some situations in which significant risks of chromosomal abnormalities have been studied by researchers. Some indications of the risks[1] are discussed below:
The instability of the chromosome is categorized at two levels: nucleotide level and chromosome level. The instability at the nucleotide level includes deletions or substitutions of a few nucleotides that cause frameshift mutations. However, instability at the chromosome level includes structural rearrangements of chromosomes (deletions, duplications, inversions, and translocation) which occurs due to breaks in the chromosomes. Another cause of chromosome instability is the numerical changes (aneuploidy and polyploidy) in the chromosome. This occurs due to the dysregulation of genes involved in cell division.
Mutations in cellular processes such as impairment of DNA replication, DNA recombination, and DNA repair lead to some other instabilities that are: the formation of fragile sites, sister chromatid exchange, and chromosome breakage.[1]
We are already familiar with the structural and numerical instability of the chromosomes. So, here we will discuss fragile sites and associated diseases.
Fragile sites are considered as the points/regions on the chromosome which form gaps or breaks when the cell is exposed to stress conditions. These sites are rare but they are inherited as codominant traits. Some of the commonly known fragile sites are 3p14.2 (FRA3B), 6q26 (FRA6E), and Xp22 (FRAXB).[1]
It has been found that fragile sites are induced by chemicals such as aphidicolin, 5-azacytidine, and bromodeoxyuridine (BrdU).
Figure: The image shows fragile sites on human chromosomes (shown by arrow)-induced by folate. Source: The Principles of Clinical Cytogenetics (2013).[1] |
The frequency of occurrence of abortion is 15-20% in the early gestation period or second and third trimester of pregnancy. Multiple studies suggested that cytogenetic abnormalities are significant factors contributing to spontaneous abortion. It has been also found that the chances of abortion or chromosomal abnormality increase with the maternal age(at the age of 35 or more). Some of the errors[1] leading to abortion are given below:
As seen, clinical cytogenetics is the study of the relation of chromosomal aberrations with genetic diseases. The study provides a deep insight into structural and numerical chromosomal abnormalities leading to variant genetic disorders. The most important aspect of clinical cytogenetics is that it helps in the analysis of fetal chromosomes for any genetic disorders and genetic counseling of parents. So, clinical cytogenetics provides a whole picture of chromosomal disorders and their causes, which proves to be a useful tool for disease diagnosis and treatment.
Gersen Steven L. and Keagle Martha B. (2013). The Principles of Clinical Cytogenetics (3rd ed.), Springer, New York.
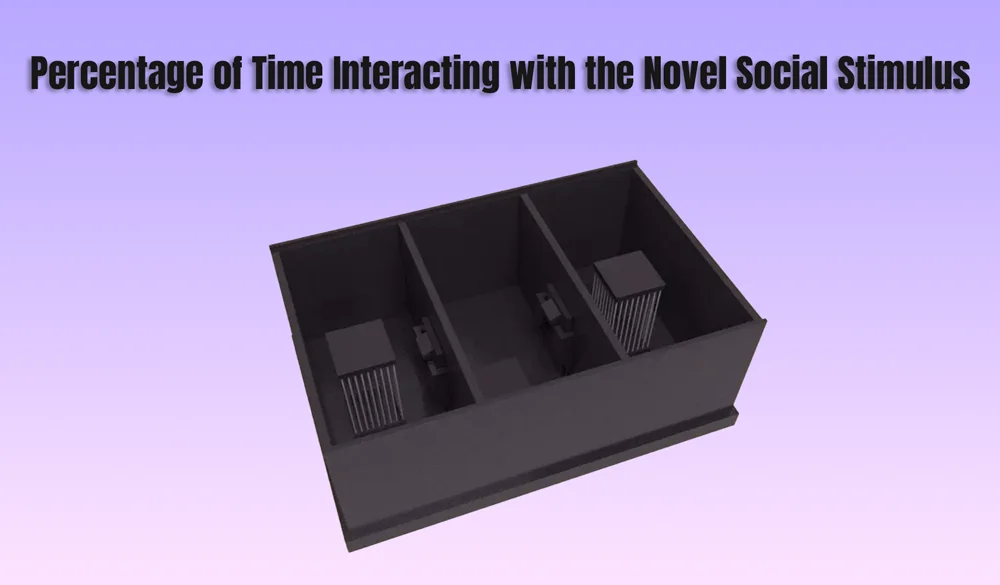
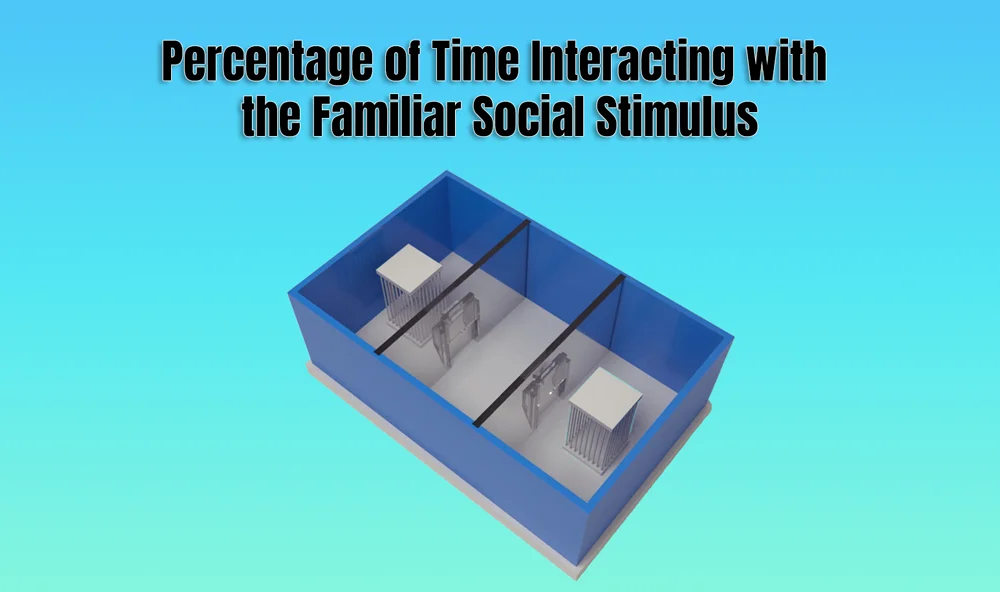
In behavioral neuroscience, the Open Field Test (OFT) remains one of the most widely used assays to evaluate rodent models of affect, cognition, and motivation. It provides a non-invasive framework for examining how animals respond to novelty, stress, and pharmacological or environmental manipulations. Among the test’s core metrics, the percentage of time spent in the center zone offers a uniquely normalized and sensitive measure of an animal’s emotional reactivity and willingness to engage with a potentially risky environment.
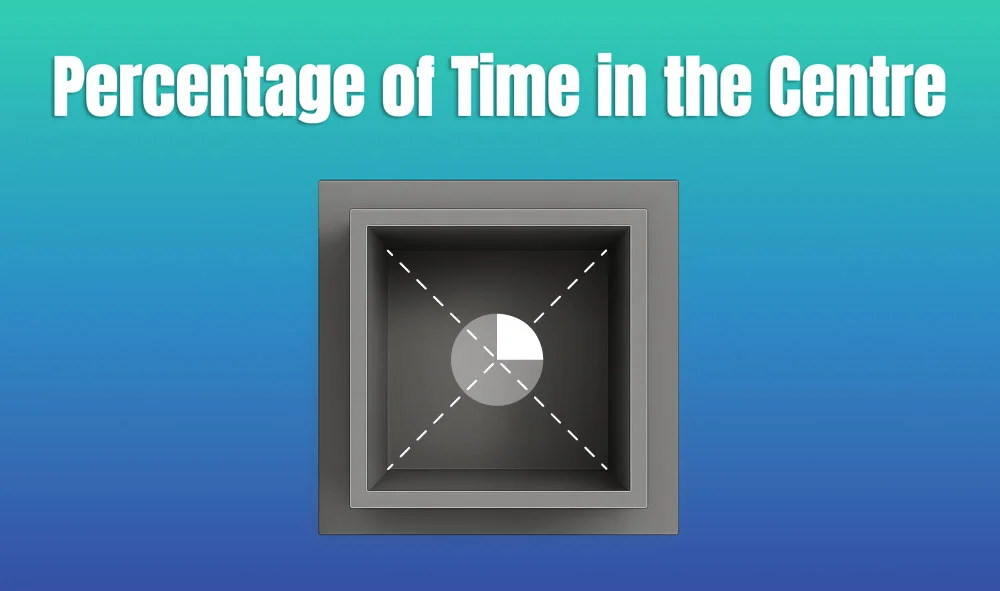
This metric is calculated as the proportion of time spent in the central area of the arena—typically the inner 25%—relative to the entire session duration. By normalizing this value, researchers gain a behaviorally informative variable that is resilient to fluctuations in session length or overall movement levels. This makes it especially valuable in comparative analyses, longitudinal monitoring, and cross-model validation.
Unlike raw center duration, which can be affected by trial design inconsistencies, the percentage-based measure enables clearer comparisons across animals, treatments, and conditions. It plays a key role in identifying trait anxiety, avoidance behavior, risk-taking tendencies, and environmental adaptation, making it indispensable in both basic and translational research contexts.
Whereas simple center duration provides absolute time, the percentage-based metric introduces greater interpretability and reproducibility, especially when comparing different animal models, treatment conditions, or experimental setups. It is particularly effective for quantifying avoidance behaviors, risk assessment strategies, and trait anxiety profiles in both acute and longitudinal designs.
This metric reflects the relative amount of time an animal chooses to spend in the open, exposed portion of the arena—typically defined as the inner 25% of a square or circular enclosure. Because rodents innately prefer the periphery (thigmotaxis), time in the center is inversely associated with anxiety-like behavior. As such, this percentage is considered a sensitive, normalized index of:
Critically, because this metric is normalized by session duration, it accommodates variability in activity levels or testing conditions. This makes it especially suitable for comparing across individuals, treatment groups, or timepoints in longitudinal studies.
A high percentage of center time indicates reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance. reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity, where animals exhibit persistent avoidance of the center due to heightened emotional reactivity. This metric can also distinguish between acute anxiety responses and enduring trait anxiety, especially in longitudinal or developmental studies. Its normalized nature makes it ideal for comparing across cohorts with variable locomotor profiles, helping researchers detect true affective changes rather than activity-based confounds.
Rodents that spend more time in the center zone typically exhibit broader and more flexible exploration strategies. This behavior reflects not only reduced anxiety but also cognitive engagement and environmental curiosity. High center percentage is associated with robust spatial learning, attentional scanning, and memory encoding functions, supported by coordinated activation in the prefrontal cortex, hippocampus, and basal forebrain. In contrast, reduced center engagement may signal spatial rigidity, attentional narrowing, or cognitive withdrawal, particularly in models of neurodegeneration or aging.
The open field test remains one of the most widely accepted platforms for testing anxiolytic and psychotropic drugs. The percentage of center time reliably increases following administration of anxiolytic agents such as benzodiazepines, SSRIs, and GABA-A receptor agonists. This metric serves as a sensitive and reproducible endpoint in preclinical dose-finding studies, mechanistic pharmacology, and compound screening pipelines. It also aids in differentiating true anxiolytic effects from sedation or motor suppression by integrating with other behavioral parameters like distance traveled and entry count (Prut & Belzung, 2003).
Sex-based differences in emotional regulation often manifest in open field behavior, with female rodents generally exhibiting higher variability in center zone metrics due to hormonal cycling. For example, estrogen has been shown to facilitate exploratory behavior and increase center occupancy, while progesterone and stress-induced corticosterone often reduce it. Studies involving gonadectomy, hormone replacement, or sex-specific genetic knockouts use this metric to quantify the impact of endocrine factors on anxiety and exploratory behavior. As such, it remains a vital tool for dissecting sex-dependent neurobehavioral dynamics.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity. Because it is normalized, this metric is especially helpful for distinguishing between genuine avoidance and low general activity.
Environmental Control: Uniformity in environmental conditions is essential. Lighting should be evenly diffused to avoid shadow bias, and noise should be minimized to prevent stress-induced variability. The arena must be cleaned between trials using odor-neutral solutions to eliminate scent trails or pheromone cues that may affect zone preference. Any variation in these conditions can introduce systematic bias in center zone behavior. Use consistent definitions of the center zone (commonly 25% of total area) to allow valid comparisons. Software-based segmentation enhances spatial precision.
Evaluating how center time evolves across the duration of a session—divided into early, middle, and late thirds—provides insight into behavioral transitions and adaptive responses. Animals may begin by avoiding the center, only to gradually increase center time as they habituate to the environment. Conversely, persistently low center time across the session can signal prolonged anxiety, fear generalization, or a trait-like avoidance phenotype.
To validate the significance of center time percentage, it should be examined alongside results from other anxiety-related tests such as the Elevated Plus Maze, Light-Dark Box, or Novelty Suppressed Feeding. Concordance across paradigms supports the reliability of center time as a trait marker, while discordance may indicate task-specific reactivity or behavioral dissociation.
When paired with high-resolution scoring of behavioral events such as rearing, grooming, defecation, or immobility, center time offers a richer view of the animal’s internal state. For example, an animal that spends substantial time in the center while grooming may be coping with mild stress, while another that remains immobile in the periphery may be experiencing more severe anxiety. Microstructure analysis aids in decoding the complexity behind spatial behavior.
Animals naturally vary in their exploratory style. By analyzing percentage of center time across subjects, researchers can identify behavioral subgroups—such as consistently bold individuals who frequently explore the center versus cautious animals that remain along the periphery. These classifications can be used to examine predictors of drug response, resilience to stress, or vulnerability to neuropsychiatric disorders.
In studies with large cohorts or multiple behavioral variables, machine learning techniques such as hierarchical clustering or principal component analysis can incorporate center time percentage to discover novel phenotypic groupings. These data-driven approaches help uncover latent dimensions of behavior that may not be visible through univariate analyses alone.
Total locomotion helps contextualize center time. Low percentage values in animals with minimal movement may reflect sedation or fatigue, while similar values in high-mobility subjects suggest deliberate avoidance. This metric helps distinguish emotional versus motor causes of low center engagement.
This measure indicates how often the animal initiates exploration of the center zone. When combined with percentage of time, it differentiates between frequent but brief visits (indicative of anxiety or impulsivity) versus fewer but sustained center engagements (suggesting comfort and behavioral confidence).
The delay before the first center entry reflects initial threat appraisal. Longer latencies may be associated with heightened fear or low motivation, while shorter latencies are typically linked to exploratory drive or low anxiety.
Time spent hugging the walls offers a spatial counterbalance to center metrics. High thigmotaxis and low center time jointly support an interpretation of strong avoidance behavior. This inverse relationship helps triangulate affective and motivational states.
By expressing center zone activity as a proportion of total trial time, researchers gain a metric that is resistant to session variability and more readily comparable across time, treatment, and model conditions. This normalized measure enhances reproducibility and statistical power, particularly in multi-cohort or cross-laboratory designs.
For experimental designs aimed at assessing anxiety, exploratory strategy, or affective state, the percentage of time spent in the center offers one of the most robust and interpretable measures available in the Open Field Test.
Written by researchers, for researchers — powered by Conduct Science.





Monday – Friday
9 AM – 5 PM EST
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.