

As an Amazon Associate Conductscience Inc earns revenue from qualifying purchases
The key parts covered in the neurological exam include:
There are twelve cranial nerves that control different aspects of facial functions. Damage to different nuclei or different sections of some nerves can lead to the presentation of different signs and symptoms.
There is little value in routinely testing olfaction as it is uncommon to detect an abnormality. Hence testing olfaction is usually omitted from a clinical exam unless there are specific clinical suspicions. A sub frontal tumor such as a meningioma may cause unilateral anosmia. Subfrontal meningioma can cause bilateral anosmia. Olfactory nerve fibers pass through the cribriform plate; hence head injury resulting in a fracture of the cribriform plate can lead to permanent bilateral anosmia. Anosmia is commonly neurodegenerative, occurring particularly in Lewy body disease. Olfaction is also tested when Kallman’s syndrome is suspected.
Testing the optic nerve consists of several components: visual acuity, color vision, visual field, pupillary examination, and fundus examination.
Visual Acuity
Acquired unilateral loss of color vision is a characteristic feature of optic neuropathy, and loss of color vision can occur even if visual acuity is intact. Hence, testing color vision using Ishihara test chart books may be a sensitive bedside test for mild optic neuropathy.
Visual field defects in one eye indicate a retinal or optic nerve defect. Lesions at the optic chiasm or lesions behind the optic chiasm in the optic tracts, visual radiations or occipital cortex will result in visual field defects in both eyes.
Visual fields are divided into nasal and temporal halves. On each side, the nasal visual field is projected to the temporal half of the retina, and the temporal visual field is projected to the nasal half of the retina. Similarly, the upper half of the visual field is projected to the lower part of the retina and vice versa. Visual field defects can occur due to lesions of various parts of the visual pathway:
Examination of the pupils and their responses to light and accommodation provides information not only about specific neurological syndromes but also information about the integrity of the anterior visual pathways, the brainstem, and the efferent parasympathetic and sympathetic pathways to the pupillary sphincter and dilator muscles, respectively. The third cranial nerve contributes the efferent portion of the visual pathway; hence the pupillary examination takes into account the pathologies of both the optic and oculomotor nerves.
Asymmetry of the pupils is referred to as anisocoria. Some people with anisocoria have no underlying neuropathology. In this setting, the asymmetry will have been present for a long time without change, and the patient will have no other neurological signs or symptoms. The direct and consensual responses should be preserved.
Some conditions can also affect the size of the pupils. Medications/ intoxications which cause generalized sympathetic activation will result in dilatation of both pupils. Other drugs (e.g., narcotics) cause symmetric constriction of the pupils. These findings can provide important clues when dealing with an agitated or comatose patient suffering from a medication overdose. Eye drops known as mydriatic agents are employed to paralyze the muscles, resulting in marked dilatation of the pupils on the affected side. They are used during a detailed eye examination, allowing a clear view of the retina. Additionally, any process which causes increased intracranial pressure can result in a dilated pupil that does not respond to light. Other conditions like anxiety, pontine lesion, and even simply old age can cause bilateral pupillary dilation.
If the afferent nerve is not working, neither pupil will respond when the light is shined in the affected eye. Light shined in the normal eye, however, will cause the affected pupil to constrict. That’s because the efferent (signal to constrict) response, in this case, is generated by the afferent impulse received by the normally functioning eye. This is referred to as an afferent pupil defect.
If the efferent nerve is not working, the pupil will appear dilated at baseline and will have neither direct nor consensual pupillary responses
These three cranial nerves control the movement of the extraocular muscles and hence are tested together. The oculomotor nerve controls the movement of the inferior oblique, inferior rectus, superior rectus, and medial rectus, along with controlling the movement of the upper lid and mediating the constriction of the pupil. The trochlear nerve controls the movement of the superior oblique. The abducent nerve controls the movement of the lateral rectus. Movements are described as elevation (pupil directed upwards), depression (pupil directed downwards), abduction (pupil directed laterally), adduction (pupil directed medially), extorsion (top of eye rotating away from the nose), and intorsion (top of eye rotating towards the nose).
Movements of the extraocular muscles:
Lateral rectus: abduction
Medial rectus: adduction
Inferior rectus: depression, extorsion, adduction
Superior rectus: elevation, intorsion, adduction
Inferior oblique: elevation, extorsion, abduction
Superior oblique: depression, intorsion, abduction
Examination
The examination of the cranial nerves should be done in such a manner that it is easy for you to observe the eye movements of the patient.
Third nerve paralysis
There is ptosis due to paralysis of levator palpebrae superioris. Ptosis can be caused by third cranial nerve palsy, Myasthenia gravis, or Horner’s syndrome.
Superior, inferior, and medial recti and inferior oblique are paralyzed. The eyeball becomes deviated laterally and slightly downwards due to the unopposed action of the superior oblique and lateral rectus. The pupil is dilated and fixed. Light and accommodation reflexes will be absent
Third nerve palsy can be caused by diabetes, aneurysm of the posterior communicating artery, midbrain lesion, or cavernous sinus thrombosis.
Fourth nerve paralysis
The superior oblique is paralyzed, leading to double vision
Sixth nerve paralysis
The lateral rectus is paralyzed, and the eyeball deviates medially. Because of its long intracranial route, the sixth cranial nerve is often involved in raised intracranial pressure of any etiology. When the patient is asked to move the eye laterally, there is nystagmus in internuclear ophthalmoplegia, and the opposite eye cannot move medially. This occurs due to the lesion in the medial longitudinal bundle on the side of the weakness of adduction. Bilateral internuclear ophthalmoplegia is characteristic of multiple sclerosis.
This is a mixed motor and sensory nerve. The neve is divided into three portions: ophthalmic division (sensory), maxillary division (sensory), and mandibular division (mixed). The motor portion supplies the muscles of mastication: masseter, temporalis, and the pterygoids. The sensory portion carries sensations of touch, pain, and temperature from the face, the anterior part of the head, and inside the mouth.
Motor examination
Sensory examination
The patient should be able to identify the sharp end touching their face. Due to the sensitivity of the face, the object should not be pushed too hard. The ophthalmic branch of CN V also receives sensory input from the surface of the eye. To assess this component the corneal and conjunctival reflexes need to be tested:
Pathology
The facial nerve innervates most of the muscles of facial expression.
Motor Function Examination
Taste Examination
The facial nerve is the most commonly affected cranial nerve by lesion of both upper motor neuron and lower motor neuron. This clinical distinction is very important, as central (UMN lesion) vs. peripheral dysfunction (LMN lesion) carry different prognostic and treatment implications. Bell’s palsy (peripheral facial nerve dysfunction) tends to happen in patients over 50 and often responds to treatment with Acyclovir (an anti-viral agent) and Prednisone (a corticosteroid). Over the course of weeks or months, there is usually improvement and often complete resolution of symptoms. Assessment of acute central facial nerve dysfunction would require quite a different approach (e.g., neuroimaging to determine etiology).
Upper Motor Neuron Lesion
Manifestations are on the opposite side. The upper half of the face is less severely affected because the part of the facial nerve nucleus which supplies muscles of the upper half of the face is connected with both cerebral hemispheres. The part of the facial nerve nucleus which supplies muscles of the lower half of the face is connected only with the contralateral cerebral hemisphere. Smiling and other emotional movements are usually preserved in UMN lesions because there is a separate path for these movements. An upper motor neuron lesion may occur with a central nervous system event, such as a stroke. The patient will present with preserved ability to wrinkle the forehead, but the corner of the mouth will deviate on the contralateral side.
Lower Motor Neuron Lesion
The whole ipsilateral half of the face is affected. Bell’s palsy is the most common cause of isolated lower motor neuron facial palsy. The lesion is in the facial canal. The facial nerve has a long route and gives off branches at various sites, the site of the lesion can be localized with considerable precision.
The vestibulocochlear nerve has two components, the cochlear component which deals with hearing, and the vestibular component which deals with a sense of balance. The examination of all these components is hence separate as well.
For the cochlear division the following tests are performed:
Whisper-Test
The Rinne’s and Weber’s tests are carried out using tuning forks, which help to differentiate between conductive deafness and sensorineural deafness.
Rinne’s Test
A positive Rinne’s test is normal. In sensory neural deafness, air conduction is better than bone conduction, but both are reduced – reduced positive Rinne’s test. In conductive deafness Rinne’s test is negative. In mixed deafness, bone conduction is better than air conduction, but both are reduced.
Weber’s Test
If it is equal on both sides, the test is central, and it indicates either normal hearing or equal deafness on both sides. In sensorineural deafness, it is lateralized to the normal ear while in conductive deafness it is lateralized to the diseased ear.
For the vestibular division the following tests are done:
Revolving Chair Test
Caloric Test
or [amazon link=”B0055QNVDM” title=”Soft Rubber Catheter” /].
The motor part of the nerve supplies the stylopharyngeus muscle and the sensory part carries sensations from the pharynx, tonsillar region, and the posterior one-third of the tongue. It also carries secretomotor fibers to the parotid gland. The motor function of the nerve cannot be tested separately from the vagus nerve hence only the sensory component examination is mentioned here.
Gag Reflex
Palatal Reflex
The vagus nerve mainly carries the parasympathetic fibers to the organs of the chest and abdomen, but there is also a motor component that supplies the muscles of the soft palate and pharynx and the intrinsic muscles of the larynx. In the examination, only the motor function of the nerve is tested.
Speech Examination
Soft palate Examination
Posterior Pharyngeal Wall Examination
The recurrent laryngeal nerve (a branch of the vagus nerve) can be damaged during thyroid surgery or by malignant tumors. Bilateral vagus nerve palsy occurs in bulbar and pseudobulbar palsy.
CN XI Accessory Nerve
The spinal accessory nerve innervates the trapezius and sternocleidomastoid muscles. The trapezius muscle is responsible for shrugging shoulders, whereas the sternocleidomastoid helps in turning the head laterally.
Examination of Sternocleidomastoid Function
Examination of Trapezius Function
The accessory nerve tends to get paralyzed along with other nerves in bulbar palsy.
The hypoglossal nerve innervates the muscles of the tongue. Each hypoglossal nerve innervates one-half of the tongue.
In bilateral UMN lesion, the tongue looks small and conical and is immobile. In unilateral UMN lesion, the tongue may sometimes deviate towards the paralyzed side when protruded. No wasting is seen in UMN lesions. In bilateral LMN lesion, there is generalized wasting with fasciculation. In unilateral LMN lesions, wasting and fasciculation are present on the affected side only.
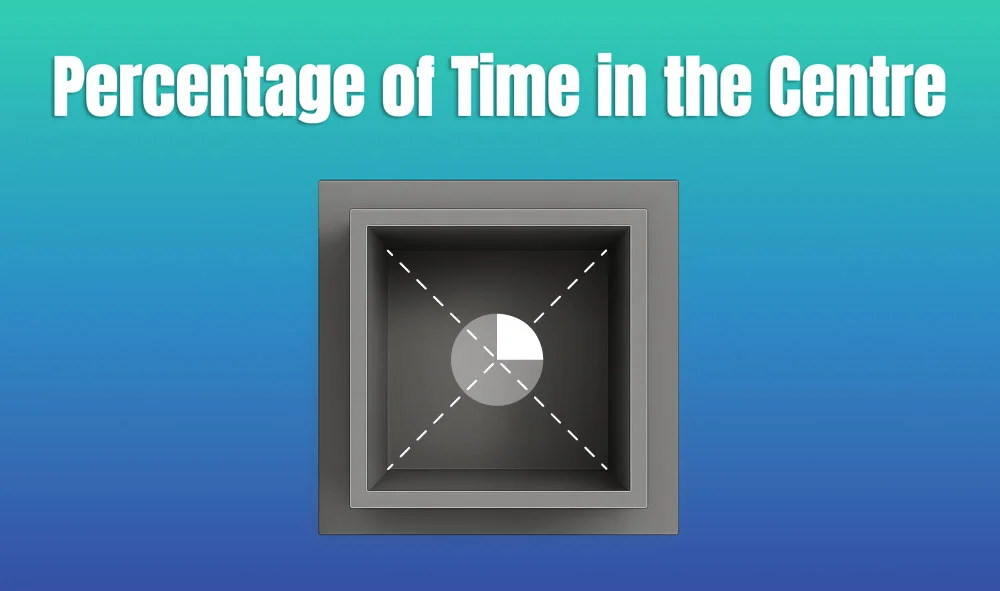
In behavioral neuroscience, the Open Field Test (OFT) remains one of the most widely used assays to evaluate rodent models of affect, cognition, and motivation. It provides a non-invasive framework for examining how animals respond to novelty, stress, and pharmacological or environmental manipulations. Among the test’s core metrics, the percentage of time spent in the center zone offers a uniquely normalized and sensitive measure of an animal’s emotional reactivity and willingness to engage with a potentially risky environment.
This metric is calculated as the proportion of time spent in the central area of the arena—typically the inner 25%—relative to the entire session duration. By normalizing this value, researchers gain a behaviorally informative variable that is resilient to fluctuations in session length or overall movement levels. This makes it especially valuable in comparative analyses, longitudinal monitoring, and cross-model validation.
Unlike raw center duration, which can be affected by trial design inconsistencies, the percentage-based measure enables clearer comparisons across animals, treatments, and conditions. It plays a key role in identifying trait anxiety, avoidance behavior, risk-taking tendencies, and environmental adaptation, making it indispensable in both basic and translational research contexts.
Whereas simple center duration provides absolute time, the percentage-based metric introduces greater interpretability and reproducibility, especially when comparing different animal models, treatment conditions, or experimental setups. It is particularly effective for quantifying avoidance behaviors, risk assessment strategies, and trait anxiety profiles in both acute and longitudinal designs.
This metric reflects the relative amount of time an animal chooses to spend in the open, exposed portion of the arena—typically defined as the inner 25% of a square or circular enclosure. Because rodents innately prefer the periphery (thigmotaxis), time in the center is inversely associated with anxiety-like behavior. As such, this percentage is considered a sensitive, normalized index of:
Critically, because this metric is normalized by session duration, it accommodates variability in activity levels or testing conditions. This makes it especially suitable for comparing across individuals, treatment groups, or timepoints in longitudinal studies.
A high percentage of center time indicates reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance. reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity, where animals exhibit persistent avoidance of the center due to heightened emotional reactivity. This metric can also distinguish between acute anxiety responses and enduring trait anxiety, especially in longitudinal or developmental studies. Its normalized nature makes it ideal for comparing across cohorts with variable locomotor profiles, helping researchers detect true affective changes rather than activity-based confounds.
Rodents that spend more time in the center zone typically exhibit broader and more flexible exploration strategies. This behavior reflects not only reduced anxiety but also cognitive engagement and environmental curiosity. High center percentage is associated with robust spatial learning, attentional scanning, and memory encoding functions, supported by coordinated activation in the prefrontal cortex, hippocampus, and basal forebrain. In contrast, reduced center engagement may signal spatial rigidity, attentional narrowing, or cognitive withdrawal, particularly in models of neurodegeneration or aging.
The open field test remains one of the most widely accepted platforms for testing anxiolytic and psychotropic drugs. The percentage of center time reliably increases following administration of anxiolytic agents such as benzodiazepines, SSRIs, and GABA-A receptor agonists. This metric serves as a sensitive and reproducible endpoint in preclinical dose-finding studies, mechanistic pharmacology, and compound screening pipelines. It also aids in differentiating true anxiolytic effects from sedation or motor suppression by integrating with other behavioral parameters like distance traveled and entry count (Prut & Belzung, 2003).
Sex-based differences in emotional regulation often manifest in open field behavior, with female rodents generally exhibiting higher variability in center zone metrics due to hormonal cycling. For example, estrogen has been shown to facilitate exploratory behavior and increase center occupancy, while progesterone and stress-induced corticosterone often reduce it. Studies involving gonadectomy, hormone replacement, or sex-specific genetic knockouts use this metric to quantify the impact of endocrine factors on anxiety and exploratory behavior. As such, it remains a vital tool for dissecting sex-dependent neurobehavioral dynamics.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity. Because it is normalized, this metric is especially helpful for distinguishing between genuine avoidance and low general activity.
Environmental Control: Uniformity in environmental conditions is essential. Lighting should be evenly diffused to avoid shadow bias, and noise should be minimized to prevent stress-induced variability. The arena must be cleaned between trials using odor-neutral solutions to eliminate scent trails or pheromone cues that may affect zone preference. Any variation in these conditions can introduce systematic bias in center zone behavior. Use consistent definitions of the center zone (commonly 25% of total area) to allow valid comparisons. Software-based segmentation enhances spatial precision.
Evaluating how center time evolves across the duration of a session—divided into early, middle, and late thirds—provides insight into behavioral transitions and adaptive responses. Animals may begin by avoiding the center, only to gradually increase center time as they habituate to the environment. Conversely, persistently low center time across the session can signal prolonged anxiety, fear generalization, or a trait-like avoidance phenotype.
To validate the significance of center time percentage, it should be examined alongside results from other anxiety-related tests such as the Elevated Plus Maze, Light-Dark Box, or Novelty Suppressed Feeding. Concordance across paradigms supports the reliability of center time as a trait marker, while discordance may indicate task-specific reactivity or behavioral dissociation.
When paired with high-resolution scoring of behavioral events such as rearing, grooming, defecation, or immobility, center time offers a richer view of the animal’s internal state. For example, an animal that spends substantial time in the center while grooming may be coping with mild stress, while another that remains immobile in the periphery may be experiencing more severe anxiety. Microstructure analysis aids in decoding the complexity behind spatial behavior.
Animals naturally vary in their exploratory style. By analyzing percentage of center time across subjects, researchers can identify behavioral subgroups—such as consistently bold individuals who frequently explore the center versus cautious animals that remain along the periphery. These classifications can be used to examine predictors of drug response, resilience to stress, or vulnerability to neuropsychiatric disorders.
In studies with large cohorts or multiple behavioral variables, machine learning techniques such as hierarchical clustering or principal component analysis can incorporate center time percentage to discover novel phenotypic groupings. These data-driven approaches help uncover latent dimensions of behavior that may not be visible through univariate analyses alone.
Total locomotion helps contextualize center time. Low percentage values in animals with minimal movement may reflect sedation or fatigue, while similar values in high-mobility subjects suggest deliberate avoidance. This metric helps distinguish emotional versus motor causes of low center engagement.
This measure indicates how often the animal initiates exploration of the center zone. When combined with percentage of time, it differentiates between frequent but brief visits (indicative of anxiety or impulsivity) versus fewer but sustained center engagements (suggesting comfort and behavioral confidence).
The delay before the first center entry reflects initial threat appraisal. Longer latencies may be associated with heightened fear or low motivation, while shorter latencies are typically linked to exploratory drive or low anxiety.
Time spent hugging the walls offers a spatial counterbalance to center metrics. High thigmotaxis and low center time jointly support an interpretation of strong avoidance behavior. This inverse relationship helps triangulate affective and motivational states.
By expressing center zone activity as a proportion of total trial time, researchers gain a metric that is resistant to session variability and more readily comparable across time, treatment, and model conditions. This normalized measure enhances reproducibility and statistical power, particularly in multi-cohort or cross-laboratory designs.
For experimental designs aimed at assessing anxiety, exploratory strategy, or affective state, the percentage of time spent in the center offers one of the most robust and interpretable measures available in the Open Field Test.
Written by researchers, for researchers — powered by Conduct Science.





Monday – Friday
9 AM – 5 PM EST
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.