

Erythrocyte sedimentation rate (ESR) is a measure of the rate of fall of RBCs at the bottom of a test tube when held upright in a fixed position for a specified time. Essentially, the ESR is a physical process that occurs in normal conditions but can increase in response to an altered blood suspension caused by numerous conditions and pathologies.
Though not precise, the ESR test serves three purposes, 1) it rules out the possibility of any underlying pathology, mainly infection and hyperinflammatory conditions (osteoarthritis, rheumatoid arthritis), 2) helps monitor disease course, and 3) is used as a screening parameter for a neoplastic pathology.
Blood response to inflammatory processes in several diseases and conditions is more or less the same. Inflammation triggers the recruitment of inflammatory mediators, which are essentially “acute phase proteins” responsible for producing the typical signs of inflammation. As the levels of the mediator proteins increase in response to inflammation, the ESR also rises simultaneously to the severity of inflammation.
A normal ESR is maintained by the reciprocal charges on the plasma proteins and membrane surfaces of RBCs. Negative charges on the RBC help separate them from each other, while positive charges on plasma proteins effectively neutralize the negative charges on erythrocytes and maintain blood physiology. However, inflammation due to an injury, stresses, and disease process raises the levels of inflammatory mediator plasma proteins, which thus disrupt the normal membrane physiology of the RBC and cause them to clump together at a faster rate.
While some pathologies or injuries cause an elevation in ESR, other conditions like Polycythemia (RBC excess) reduce ESR. A reduced ESR may result from an isolated disease process or may accompany an underlying severe illness that must raise the ESR but instead produces ESR values lower than expected.
ESR is measured using an automated analyzer or with manual methods. However, ESR values measured with the manual methods are sensitive to changes in temperature and vibrations. Therefore, it is essential to consider and maintain the two factors when conducting the ESR test. Another way to measure the ESR is to directly evaluate the levels of plasma proteins.
The two most common manual methods used by labs, specifically animal labs, to obtain the ESR are the Wintrobe and Westergren methods. Both the methods are relatively similar. The Westergren method is conducted with the 200 mm long Westergren-Katz tube, whereas the Wintrobe method is conducted with the 100 mm long and shorter diameter Wintrobe tube.
In the Westergren method, a 200 mm tube containing 0.5 ml of anticoagulant sodium citrate is used to collect 2 ml of venous blood from the subject. The collected blood should be used within 2 hours at room temperature or 6 hours if kept at 4 degrees Celsius. To calculate the ESR, the blood tube is held upright in a rack for 1 hour to allow the RBCs to sediment at the bottom of the tube and the distance from the lowest point of plasma meniscus to the RBC sediment is noted.
In the Wintrobe Method, a smaller diameter 100 mm tube containing EDTA anticoagulant is used to collect venous blood. Once collected, the blood tube is held in a vertical position for 1 hour to permit RBC collection at the bottom, and the ESR is measured in the same manner as done for the Westergren method. Due to the limited length of the Wintrobe tube (100 mm), ESR measurements are subject to inaccuracy and abnormal readings. Essentially, this method is convenient for demonstration purposes only.
The principle for ESR testing is based on the differences in densities of plasma proteins and RBCs. Since RBCs are denser than plasma proteins, they tend to settle at the bottom if kept at rest in a tube while the plasma collects above the RBC aggregate as supernatant.
In normal conditions, RBCs carry negative charges on their membrane surfaces that keep them apart from each other and prevent clumping into aggregates (rouleaux formation). In contrast, plasma proteins carry positive charges to neutralize the negatively charged erythrocytes. Based on current concepts in hematology, it is believed that an increase in plasma proteins called agglomerans (fibrinogen, IgM, and alpha 2 macroglobulins) and changes in the RBC membrane are manifestations of inflammation, pregnancy, malignancy, and infection.
As inflammation ensues, levels of positively charged plasma proteins increase to the point that the negative charges on erythrocytes are no longer strong enough to repel other erythrocytes. Consequently, RBCs lose their ability to stay apart and clump to form aggregates.
The disease processes cause changes in the erythrocyte membrane by altering the distribution of charges on RBC surfaces, encouraging their tendency to clump together, and thereby raising the erythrocyte sedimentation rate (ESR).
ESR is utilized as a general index to ascertain the existing illness or pathology. The blood ESR is measured for the subject with a suspected pathology. However, the ESR merely confirms the presence of the pathology but does not denote the severity or identification of the disease.
First, sterilize and prepare blood collection tubes by adding the appropriate anticoagulant to each. Carefully fixate the animal in restrainer and expose the puncture site. Rub the puncture site with an alcohol swab before inserting the needle.
Once the needle enters the puncture site, pull back the syringe plunger to collect blood as required and clean the puncture site right after drawing out the blood. Only apply gentle force on the plunger to release the collected blood in the anticoagulant-containing tube as it is vital to avoid RBC damage and hemolysis. Store the tubes in an appropriate container and then examine for any blood clots after some time.
For a standard tube, invert the tube at least 10-12 times for every 5ml blood collected and for a non-standard, especially narrow tube, more than 12 times to exclude air bubbles. Continue mixing the blood until immediately before inserting the pipette into the blood tube.
Adjust the blood column or scale of the pipette to allow correction for minimal variation in the apparent volume. Insert the Westergren or Wintrobe pipettes (48,49) in blood tubes to collect the blood in the pipette to mark 0 without including air bubbles. Then, hold the pipette in a fixed vertical position for 1 hour at room temperature or ambient temperature (18-25 degrees Celsius) to let the RBCs settle at the bottom. Ensure to place the pipette holder away from direct sunlight and on a sturdy, immobile table or base to prevent vibrations and drafts. Also, consider conducting ESR measurements in a temperature-controlled room.
Once an hour has passed, record the per mm fall of the RBCs in that hour and note down the resultant value as this is the erythrocyte sedimentation rate for the specified blood sample. To record the mm fall of the RBC per hour, read the distance from the surface of sedimented erythrocytes and the base of the plasma membrane meniscus. Carefully examine the reading and exclude any visible leukocyte collection (buffy coat) usually seen with the RBC column.
Applications of ESR testing in animal laboratories have been minimized due to the advent of more specific tests. These include the evaluation of inflammatory biomarkers, particularly the acute phase proteins and C reactive protein in the blood. However, ESR is still an effective parameter used to examine the presence of inflammation in several conditions and diseases in animals.
In numerous diagnostic findings, raised ESR and NO (nitric oxide) levels have been associated with rheumatological diseases. The cause of the simultaneous rise in ESR and Nitric oxide levels is yet to be discovered. However, it is postulated that inflammation triggers the production of nitric oxide, which is a key biomarker found in inflammatory conditions. At the same time, inflammation is associated with elevated ESR levels. (Uzun, M., et al. 2008).
Uzun et al. (2008) tested two groups of healthy rabbits injected with nitric oxide enzymes L- Arginine, and Nitroarginine methyl ester (L-NAME). They then evaluated the resultant effect on ESR levels. They injected the L arginine and L- NAME intraperitoneally and then collected blood samples from each rabbit via the marginal ear vein in anticoagulant-containing tubes. First, NOx levels in the blood were determined with a colorimeter, and then blood was collected in Westergren tubes to note down the per mm fall of RBCs (ESR) at 30min, 60 min, and 120min. Results obtained from the control, the L-Arginine and L-NAME groups exhibited a positive correlation between the NOx and ESR levels in the blood.
In another study, Salimi-Asl et al, (2016) evaluated the role of inflammation as a possible etiology in PCOS (polycystic Ovarian Syndrome). They studied PCOS female mice models by injecting the pubertal growth hormone (DHEA) subcutaneously in 26 prepubertal female mice for 20 days consecutively. On the 21st day, ESR values were determined after 1 hr for the blood collected in the micro dispense tubes. The ESR values for mice injected with DHEA were significantly higher than the control group, which suggested that low-grade chronic inflammation may have led to the development of PCOS.
One necessary factor to be considered during ESR measurement is that blood tubes stored at ambient temperatures (18-25 degrees Celsius) must be utilized within 4 hours for ESR. Blood tubes stored at 4 degrees Celsius can reproduce acceptable ESR values if used within 12 hours. However, stable ESR values have also been recorded after storage for up to 24 hours at 4 degrees Celsius.
ESR test serves as a general index that helps determine the likelihood of a suspected pathology in blood. While it may not determine the disease, ESR can be used to monitor and screen pathologies that trigger inflammatory pathways in the blood.
Both Wintrobe and Westergren methods of ESR Testing are conducted with minimal equipment, including blood collection syringes, tubes, and pipettes for ESR measurement. Moreover, ESR is time-efficient as it yields results quickly due to its simple protocol.
It is a widely known fact that ESR tests are less sensitive and specific than newer, highly accurate blood tests like ELISA and others. Though common in diagnostic labs, ESR only provides a general clue of the existent inflammation, so additional tests must be conducted to establish a diagnosis.
At times, the ESR values may not coincide with the accurate picture and severity of the existing pathology. For instance, in polycythemia, the ESR values are reduced due to increased blood viscosity (erythrocyte excess).
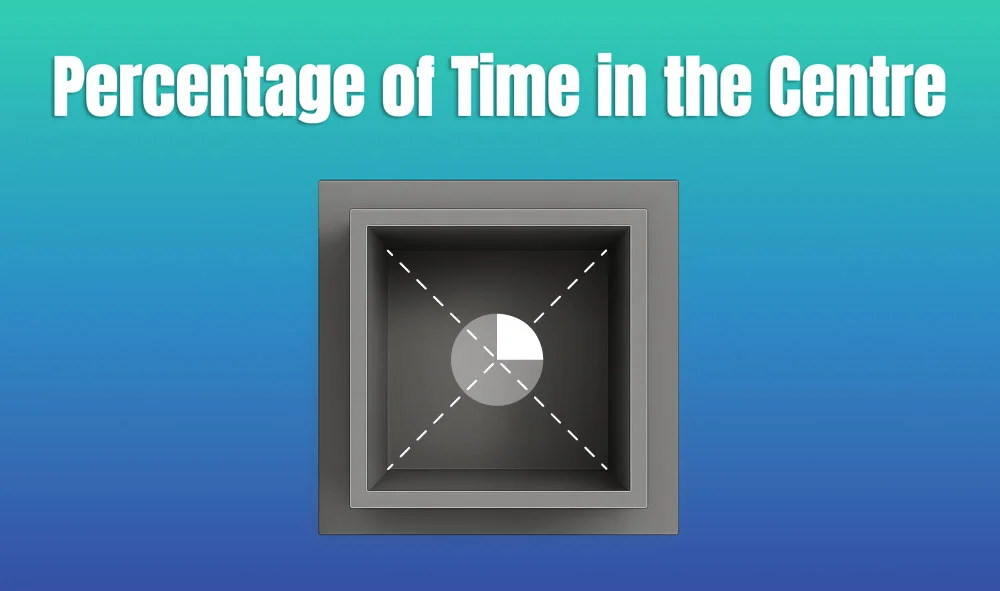
In behavioral neuroscience, the Open Field Test (OFT) remains one of the most widely used assays to evaluate rodent models of affect, cognition, and motivation. It provides a non-invasive framework for examining how animals respond to novelty, stress, and pharmacological or environmental manipulations. Among the test’s core metrics, the percentage of time spent in the center zone offers a uniquely normalized and sensitive measure of an animal’s emotional reactivity and willingness to engage with a potentially risky environment.
This metric is calculated as the proportion of time spent in the central area of the arena—typically the inner 25%—relative to the entire session duration. By normalizing this value, researchers gain a behaviorally informative variable that is resilient to fluctuations in session length or overall movement levels. This makes it especially valuable in comparative analyses, longitudinal monitoring, and cross-model validation.
Unlike raw center duration, which can be affected by trial design inconsistencies, the percentage-based measure enables clearer comparisons across animals, treatments, and conditions. It plays a key role in identifying trait anxiety, avoidance behavior, risk-taking tendencies, and environmental adaptation, making it indispensable in both basic and translational research contexts.
Whereas simple center duration provides absolute time, the percentage-based metric introduces greater interpretability and reproducibility, especially when comparing different animal models, treatment conditions, or experimental setups. It is particularly effective for quantifying avoidance behaviors, risk assessment strategies, and trait anxiety profiles in both acute and longitudinal designs.
This metric reflects the relative amount of time an animal chooses to spend in the open, exposed portion of the arena—typically defined as the inner 25% of a square or circular enclosure. Because rodents innately prefer the periphery (thigmotaxis), time in the center is inversely associated with anxiety-like behavior. As such, this percentage is considered a sensitive, normalized index of:
Critically, because this metric is normalized by session duration, it accommodates variability in activity levels or testing conditions. This makes it especially suitable for comparing across individuals, treatment groups, or timepoints in longitudinal studies.
A high percentage of center time indicates reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance. reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity, where animals exhibit persistent avoidance of the center due to heightened emotional reactivity. This metric can also distinguish between acute anxiety responses and enduring trait anxiety, especially in longitudinal or developmental studies. Its normalized nature makes it ideal for comparing across cohorts with variable locomotor profiles, helping researchers detect true affective changes rather than activity-based confounds.
Rodents that spend more time in the center zone typically exhibit broader and more flexible exploration strategies. This behavior reflects not only reduced anxiety but also cognitive engagement and environmental curiosity. High center percentage is associated with robust spatial learning, attentional scanning, and memory encoding functions, supported by coordinated activation in the prefrontal cortex, hippocampus, and basal forebrain. In contrast, reduced center engagement may signal spatial rigidity, attentional narrowing, or cognitive withdrawal, particularly in models of neurodegeneration or aging.
The open field test remains one of the most widely accepted platforms for testing anxiolytic and psychotropic drugs. The percentage of center time reliably increases following administration of anxiolytic agents such as benzodiazepines, SSRIs, and GABA-A receptor agonists. This metric serves as a sensitive and reproducible endpoint in preclinical dose-finding studies, mechanistic pharmacology, and compound screening pipelines. It also aids in differentiating true anxiolytic effects from sedation or motor suppression by integrating with other behavioral parameters like distance traveled and entry count (Prut & Belzung, 2003).
Sex-based differences in emotional regulation often manifest in open field behavior, with female rodents generally exhibiting higher variability in center zone metrics due to hormonal cycling. For example, estrogen has been shown to facilitate exploratory behavior and increase center occupancy, while progesterone and stress-induced corticosterone often reduce it. Studies involving gonadectomy, hormone replacement, or sex-specific genetic knockouts use this metric to quantify the impact of endocrine factors on anxiety and exploratory behavior. As such, it remains a vital tool for dissecting sex-dependent neurobehavioral dynamics.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity. Because it is normalized, this metric is especially helpful for distinguishing between genuine avoidance and low general activity.
Environmental Control: Uniformity in environmental conditions is essential. Lighting should be evenly diffused to avoid shadow bias, and noise should be minimized to prevent stress-induced variability. The arena must be cleaned between trials using odor-neutral solutions to eliminate scent trails or pheromone cues that may affect zone preference. Any variation in these conditions can introduce systematic bias in center zone behavior. Use consistent definitions of the center zone (commonly 25% of total area) to allow valid comparisons. Software-based segmentation enhances spatial precision.
Evaluating how center time evolves across the duration of a session—divided into early, middle, and late thirds—provides insight into behavioral transitions and adaptive responses. Animals may begin by avoiding the center, only to gradually increase center time as they habituate to the environment. Conversely, persistently low center time across the session can signal prolonged anxiety, fear generalization, or a trait-like avoidance phenotype.
To validate the significance of center time percentage, it should be examined alongside results from other anxiety-related tests such as the Elevated Plus Maze, Light-Dark Box, or Novelty Suppressed Feeding. Concordance across paradigms supports the reliability of center time as a trait marker, while discordance may indicate task-specific reactivity or behavioral dissociation.
When paired with high-resolution scoring of behavioral events such as rearing, grooming, defecation, or immobility, center time offers a richer view of the animal’s internal state. For example, an animal that spends substantial time in the center while grooming may be coping with mild stress, while another that remains immobile in the periphery may be experiencing more severe anxiety. Microstructure analysis aids in decoding the complexity behind spatial behavior.
Animals naturally vary in their exploratory style. By analyzing percentage of center time across subjects, researchers can identify behavioral subgroups—such as consistently bold individuals who frequently explore the center versus cautious animals that remain along the periphery. These classifications can be used to examine predictors of drug response, resilience to stress, or vulnerability to neuropsychiatric disorders.
In studies with large cohorts or multiple behavioral variables, machine learning techniques such as hierarchical clustering or principal component analysis can incorporate center time percentage to discover novel phenotypic groupings. These data-driven approaches help uncover latent dimensions of behavior that may not be visible through univariate analyses alone.
Total locomotion helps contextualize center time. Low percentage values in animals with minimal movement may reflect sedation or fatigue, while similar values in high-mobility subjects suggest deliberate avoidance. This metric helps distinguish emotional versus motor causes of low center engagement.
This measure indicates how often the animal initiates exploration of the center zone. When combined with percentage of time, it differentiates between frequent but brief visits (indicative of anxiety or impulsivity) versus fewer but sustained center engagements (suggesting comfort and behavioral confidence).
The delay before the first center entry reflects initial threat appraisal. Longer latencies may be associated with heightened fear or low motivation, while shorter latencies are typically linked to exploratory drive or low anxiety.
Time spent hugging the walls offers a spatial counterbalance to center metrics. High thigmotaxis and low center time jointly support an interpretation of strong avoidance behavior. This inverse relationship helps triangulate affective and motivational states.
By expressing center zone activity as a proportion of total trial time, researchers gain a metric that is resistant to session variability and more readily comparable across time, treatment, and model conditions. This normalized measure enhances reproducibility and statistical power, particularly in multi-cohort or cross-laboratory designs.
For experimental designs aimed at assessing anxiety, exploratory strategy, or affective state, the percentage of time spent in the center offers one of the most robust and interpretable measures available in the Open Field Test.
Written by researchers, for researchers — powered by Conduct Science.





Monday – Friday
9 AM – 5 PM EST
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.