

Ion Exchange Chromatography (IEC) is a powerful liquid chromatographic technique used for bioseparation. The separation is done by a reversible interaction between charged molecules of the sample with charged ligands attached to a column. The method offers a sizeable sample-handling capacity, powerful resolving ability, broad applicability, moderate cost, and ease of handling. These characteristics have made ion-exchange chromatography one of the most versatile and widely used liquid chromatography techniques. Ion exchange chromatography is frequently used for the separation and purification of polypeptides, proteins, enzymes, antibodies, nucleic acids, polynucleotides, and other charged biomolecules.
There are two types of ion-exchange chromatography: anion-exchange and cation-exchange. Cation-exchange chromatography is used for the positively charged molecules. In this type of chromatography, the stationary phase is negatively charged, which attracts the positively charged molecules of interest. Whereas, in anion-exchange chromatography, the stationary phase is positively charged which attracts the negatively charged molecules. It is prominently used in protein purification, water analysis, and quality control. The bound molecules are eluted and collected using an eluant containing anions and cations by running a higher concentration of ions through the column or changing the pH of the column.
Ion-exchange chromatography separates the molecules based on their respective charged groups. The ion-exchange chromatography consists of both mobile and stationary phases, the former is usually an aqueous buffer system into which the sample of interest is introduced, and the latter is an inert organic matrix chemically derived with ionizable functional groups carrying an oppositely charged counterion. The molecules undergo electrostatic interactions with opposite charges present on the stationary phase matrix. For electroneutrality, these inert charges interact with exchangeable counterions in the solution. Ionizable molecules that are to be resolved to compete with these counterions for binding to the displaceable charges on the stationary phase. These molecules are retained or eluted according to their respective charges.
The ion-exchange chromatography consists of a series of mobile phase reservoirs containing a range of different mobile phases. The reservoirs are made of glass or plastic because the mobile phases can have extreme pH values. The components of the ion-chromatography system include pumps, conduits, valves, sampling devices, columns, and detector cells. The solvent reservoirs are attached with a solvent selection valve and a solvent programmer. The solvent then passes from the selector to a high-pressure pump. The mobile phase passes to the sampling device from the pump and relays onto the column. The exit flow from the column passes the solvent to the detector. The detector could be an electrical conductivity detector or the UV detector. The output from the detector sensor is electronically modified and presents the ion concentration on the computer.
Sample preparation
Resin equilibration
Chromatography
Preparation of bovine cytosolic extract
Ammonium sulfate precipitation
Partial purification of Prolyl Oligopeptidase
Post-DEAE fractions assay
Analysis of the components of a Clostridium difficile vaccine (Rustandi. et al., 2016)
The ion-exchange chromatography was used to characterize the charged variant heterogeneities and to monitor the stability of antigenic components of the Clostridium difficile vaccine. In the study, a novel tetravalent C. difficile vaccine containing all four toxins was developed from an insect cell expression system. Clostridium difficile is the leading pathogen causing nosocomial diarrhea. The main virulence factors are two large glucosyltransferase proteins, toxin A (TcdA) and toxin B (TcdB). These factors are considered to be the primary factors responsible for the symptoms of the infection. The ion-exchange chromatography is a powerful separation technique for the characterization and analysis of protein antigenic components.
Determination of the protein concentration (Hugli. & Moore., 1972)
The ion-exchange chromatography was used for quantitative recoveries of tryptophan on an automatic analyzer. The proteins were hydrolyzed at 110o or 135o in NaOH containing 25 mg of starch. In the study, n-tryptophan was chromatographed on PA-35 resin and yielded values for carbon, hydrogen, and nitrogen within 1.0% of the calculated values. Integral values were obtained for the tryptophan residues in tryptophyl-leucine, porcine pepsin, human serum albumin, sperm whale apomyoglobin, trypsin, bovine a-chymotrypsin, deoxyribonuclease, and serum albumin. The method is advantageous for the measurement of tryptophan in the same hydrolysate used for the determination of the other amino acids. The ion-exchange chromatography could be efficiently used for the determination of amino acid content in hydrolysates.
Characterization of biopharmaceuticals (Fekete., Beck., Veuthey., & Guillarme., 2015)
Ion-exchange chromatography is widely used for the detailed characterization of therapeutic compounds and their derivatives. It can be considered for the qualitative and quantitative assessment of charge heterogeneity of the pharmaceutical agents. The technique has been used for the evaluation of monoclonal antibodies (Mabs), antibody drug conjugates (ADCs), polypeptides, proteins, nucleotides, and nucleic acid conjugates. The ion-exchange chromatography has been employed to separate a wide array of therapeutic agents including C-terminal lysine variants/truncation, deamidated forms, glycoforms, and sialic acid variants. In addition, it also isolates the products of the PEGylation reaction based on the degree of conjugation and the isomeric forms of PEGylated proteins. The method is a promising separation and characterization tool for the analysis of biotherapeutics.
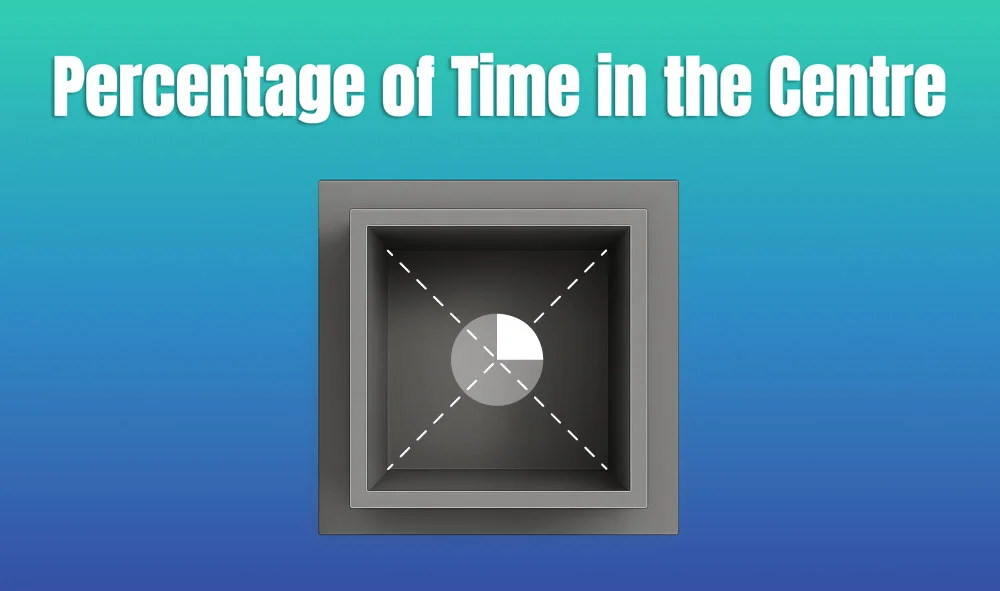
In behavioral neuroscience, the Open Field Test (OFT) remains one of the most widely used assays to evaluate rodent models of affect, cognition, and motivation. It provides a non-invasive framework for examining how animals respond to novelty, stress, and pharmacological or environmental manipulations. Among the test’s core metrics, the percentage of time spent in the center zone offers a uniquely normalized and sensitive measure of an animal’s emotional reactivity and willingness to engage with a potentially risky environment.
This metric is calculated as the proportion of time spent in the central area of the arena—typically the inner 25%—relative to the entire session duration. By normalizing this value, researchers gain a behaviorally informative variable that is resilient to fluctuations in session length or overall movement levels. This makes it especially valuable in comparative analyses, longitudinal monitoring, and cross-model validation.
Unlike raw center duration, which can be affected by trial design inconsistencies, the percentage-based measure enables clearer comparisons across animals, treatments, and conditions. It plays a key role in identifying trait anxiety, avoidance behavior, risk-taking tendencies, and environmental adaptation, making it indispensable in both basic and translational research contexts.
Whereas simple center duration provides absolute time, the percentage-based metric introduces greater interpretability and reproducibility, especially when comparing different animal models, treatment conditions, or experimental setups. It is particularly effective for quantifying avoidance behaviors, risk assessment strategies, and trait anxiety profiles in both acute and longitudinal designs.
This metric reflects the relative amount of time an animal chooses to spend in the open, exposed portion of the arena—typically defined as the inner 25% of a square or circular enclosure. Because rodents innately prefer the periphery (thigmotaxis), time in the center is inversely associated with anxiety-like behavior. As such, this percentage is considered a sensitive, normalized index of:
Critically, because this metric is normalized by session duration, it accommodates variability in activity levels or testing conditions. This makes it especially suitable for comparing across individuals, treatment groups, or timepoints in longitudinal studies.
A high percentage of center time indicates reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance. reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity, where animals exhibit persistent avoidance of the center due to heightened emotional reactivity. This metric can also distinguish between acute anxiety responses and enduring trait anxiety, especially in longitudinal or developmental studies. Its normalized nature makes it ideal for comparing across cohorts with variable locomotor profiles, helping researchers detect true affective changes rather than activity-based confounds.
Rodents that spend more time in the center zone typically exhibit broader and more flexible exploration strategies. This behavior reflects not only reduced anxiety but also cognitive engagement and environmental curiosity. High center percentage is associated with robust spatial learning, attentional scanning, and memory encoding functions, supported by coordinated activation in the prefrontal cortex, hippocampus, and basal forebrain. In contrast, reduced center engagement may signal spatial rigidity, attentional narrowing, or cognitive withdrawal, particularly in models of neurodegeneration or aging.
The open field test remains one of the most widely accepted platforms for testing anxiolytic and psychotropic drugs. The percentage of center time reliably increases following administration of anxiolytic agents such as benzodiazepines, SSRIs, and GABA-A receptor agonists. This metric serves as a sensitive and reproducible endpoint in preclinical dose-finding studies, mechanistic pharmacology, and compound screening pipelines. It also aids in differentiating true anxiolytic effects from sedation or motor suppression by integrating with other behavioral parameters like distance traveled and entry count (Prut & Belzung, 2003).
Sex-based differences in emotional regulation often manifest in open field behavior, with female rodents generally exhibiting higher variability in center zone metrics due to hormonal cycling. For example, estrogen has been shown to facilitate exploratory behavior and increase center occupancy, while progesterone and stress-induced corticosterone often reduce it. Studies involving gonadectomy, hormone replacement, or sex-specific genetic knockouts use this metric to quantify the impact of endocrine factors on anxiety and exploratory behavior. As such, it remains a vital tool for dissecting sex-dependent neurobehavioral dynamics.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity. Because it is normalized, this metric is especially helpful for distinguishing between genuine avoidance and low general activity.
Environmental Control: Uniformity in environmental conditions is essential. Lighting should be evenly diffused to avoid shadow bias, and noise should be minimized to prevent stress-induced variability. The arena must be cleaned between trials using odor-neutral solutions to eliminate scent trails or pheromone cues that may affect zone preference. Any variation in these conditions can introduce systematic bias in center zone behavior. Use consistent definitions of the center zone (commonly 25% of total area) to allow valid comparisons. Software-based segmentation enhances spatial precision.
Evaluating how center time evolves across the duration of a session—divided into early, middle, and late thirds—provides insight into behavioral transitions and adaptive responses. Animals may begin by avoiding the center, only to gradually increase center time as they habituate to the environment. Conversely, persistently low center time across the session can signal prolonged anxiety, fear generalization, or a trait-like avoidance phenotype.
To validate the significance of center time percentage, it should be examined alongside results from other anxiety-related tests such as the Elevated Plus Maze, Light-Dark Box, or Novelty Suppressed Feeding. Concordance across paradigms supports the reliability of center time as a trait marker, while discordance may indicate task-specific reactivity or behavioral dissociation.
When paired with high-resolution scoring of behavioral events such as rearing, grooming, defecation, or immobility, center time offers a richer view of the animal’s internal state. For example, an animal that spends substantial time in the center while grooming may be coping with mild stress, while another that remains immobile in the periphery may be experiencing more severe anxiety. Microstructure analysis aids in decoding the complexity behind spatial behavior.
Animals naturally vary in their exploratory style. By analyzing percentage of center time across subjects, researchers can identify behavioral subgroups—such as consistently bold individuals who frequently explore the center versus cautious animals that remain along the periphery. These classifications can be used to examine predictors of drug response, resilience to stress, or vulnerability to neuropsychiatric disorders.
In studies with large cohorts or multiple behavioral variables, machine learning techniques such as hierarchical clustering or principal component analysis can incorporate center time percentage to discover novel phenotypic groupings. These data-driven approaches help uncover latent dimensions of behavior that may not be visible through univariate analyses alone.
Total locomotion helps contextualize center time. Low percentage values in animals with minimal movement may reflect sedation or fatigue, while similar values in high-mobility subjects suggest deliberate avoidance. This metric helps distinguish emotional versus motor causes of low center engagement.
This measure indicates how often the animal initiates exploration of the center zone. When combined with percentage of time, it differentiates between frequent but brief visits (indicative of anxiety or impulsivity) versus fewer but sustained center engagements (suggesting comfort and behavioral confidence).
The delay before the first center entry reflects initial threat appraisal. Longer latencies may be associated with heightened fear or low motivation, while shorter latencies are typically linked to exploratory drive or low anxiety.
Time spent hugging the walls offers a spatial counterbalance to center metrics. High thigmotaxis and low center time jointly support an interpretation of strong avoidance behavior. This inverse relationship helps triangulate affective and motivational states.
By expressing center zone activity as a proportion of total trial time, researchers gain a metric that is resistant to session variability and more readily comparable across time, treatment, and model conditions. This normalized measure enhances reproducibility and statistical power, particularly in multi-cohort or cross-laboratory designs.
For experimental designs aimed at assessing anxiety, exploratory strategy, or affective state, the percentage of time spent in the center offers one of the most robust and interpretable measures available in the Open Field Test.
Written by researchers, for researchers — powered by Conduct Science.





Monday – Friday
9 AM – 5 PM EST
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.