

Enumeration of bacteria is defined as the process of determining the number of bacteria in a given sample.
There are numerous reasons why researchers have to calculate the number of bacteria or compare the growing number of these microbes under certain specific conditions. It’s mainly essential and a part of routine work in food, water, and dairy microbiology labs.[1]
The knowledge of the numbers of these microorganisms in our food, including milk, curd, buttermilk, and water, help the labs to determine if the prepared food or available water is hygienic or not for consumption.
However, as we know, these microorganisms are so small in size and are invisible to our naked eyes; thus, a high-power microscope is required to study these organisms. Hence, counting them is often a pain in the neck.
But thankfully, scientists have developed high-throughput protocols to make it easier to count the numbers of these organisms in any given sample.
This article discusses the techniques used to count bacteria in food or water samples and the advantages and disadvantages of these microbiological techniques.
Bacteria enumeration is divided into four categories:[2]
This process counts the number of bacteria in a liquid media or colonies on a plate. In this process, counting chambers is used to directly count the bacterial numbers under the microscope.
It’s the process of estimation of bacterial numbers. This process doesn’t involve direct counting of bacterial cell numbers, however, bacterial colonies are calculated through which the concentration of bacterial cells is estimated in the given sample. It’s done by the plate count method.
It’s counting bacterial cells that are metabolically active and actively dividing and growing in the culture medium.
Involves counting the total bacterial cell numbers, including both metabolically active and metabolically inactive/dead microbes.[2]
These four categories of estimating bacteria population are combined in four ways based on the purpose of experimentation in labs. It includes:[2]
The determination of bacterial cell numbers can be achieved by many methods, including culture turbidity, dry weight of cells, and direct microscopic count. However, among these, the direct viable count technique is the one that’s commonly used.[3]
Below are some frequently used bacteria enumeration techniques, their principles, and their differences.
Bacteria can be present in thousands or millions in a sample, making it difficult to count their numbers. However, serially diluting the cultures makes it easier to determine the count.
After serial dilution, the aliquots of the diluted sample are plated on an appropriate culture media. Then, the plates are incubated, after which the number of colonies formed is counted. This technique is also known as plate count or colony counts.[3]
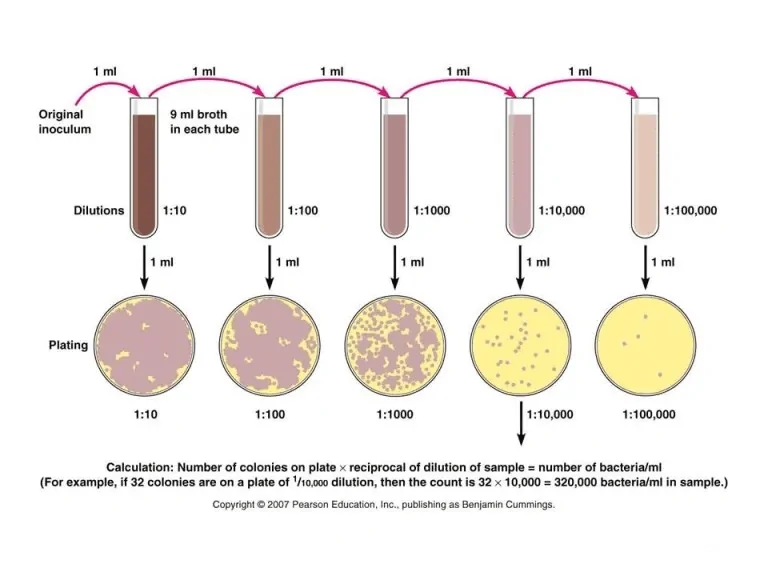
The techniques that assist in standard plate count include the streak plate and pour plate methods. It’s essential to ensure that the plate is not crowded with bacterial colonies because, in such conditions, some cells might either not form colonies or fuse with each other resulting in erroneous results. Statistically, it is most valid to count colonies only on plates with 30 to 300 colonies.[3]
In this method, accurate determination of total cell numbers is only possible if each colony is formed of a single cell. However, it’s difficult for one to ensure such a case; that’s why the total numbers of cells reported using this method are termed Colony Forming Units (CFUs) rather than cell numbers. It’s calculated as:[3]
The number of CFUs per ml of sample = The number of colonies (30-300 plate) X The dilution factor of the plate counted.
Turbidity measures the loss of intensity of transmitted light due to the scattering effect of suspended particles.[5] It’s simply the amount of cloudiness or turbidity in the sample. It can be caused by silt, sand, mud bacteria, and other microbes or chemical precipitates.
The turbidimetric method is a quick and efficient method to measure and estimate the number of bacteria in a given sample. The method is most preferable when large numbers of cultures are required to be counted.
When bacteria are mixed in a liquid medium, it creates a colloidal suspension that blocks and reflects light as it passes through the culture. The light absorbed by bacterial suspension will estimate the concentration of bacteria in the given sample.
You must note that “the light absorbed by the culture is directly proportional to the cells’ concentration.”[5]
Even though the turbidity measurement technique is faster than the standard plate count, it is initially required to match the measurement values with cell numbers, which requires lab personnel to combine the standard plate count method with the turbidity technique. The procedure followed include:[5]
Once drawn, the standard graph can be directly used for subsequent turbidity measurement and calculating the number of viable bacterial cells, eliminating the process of time-consuming standard plate count.
The method utilize instruments such as a spectrophotometer or colorimeter to measure the turbidity in the given sample. They contain a light source and a light detector separated from the sample compartment.[3]
The cultures are poured into a cuvette and kept in the instrument. When light passes through the culture sample, cells interfere with them, which provides an output value on the machine that helps to calculate the bacteria numbers in the cultures.
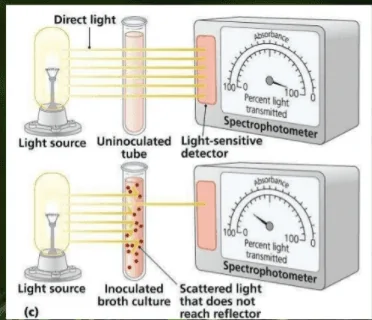
Before experimenting, it’s essential to adjust the spectrophotometer at 100% transmittance (which means 0% absorbance), which is done using a sample of the uninoculated medium.
At last, the percentage of transmittance of different bacterial culture dilutions is measured, and the obtained values are converted to optical density using the formula:[1]
Absorbance (O.D.) = 2 – log % Transmittance
The wavelength is determined based on the color of the solution. For example, if it’s white, 420 nm is used, 540 nm if the color is yellow[1], and 600-625 nm if the solution is in the range of yellow to brown color.
The direct microscopic count is used for quantitative enumeration of bacteria in water, food, milk, and air samples.[3]
It is done by spreading a measured volume of the sample over a predetermined area of a slide, counting representative microscopic fields, and transforming the average values into appropriate volume-area factors.[3]
The counting chambers used for the procedures include Petroff-Hauser and Levy counting chambers.[3]
The Petroff-Hauser is a thick microscope slide with a chamber in the middle of 0.02 mm (1/50 mm) deep, and it also has improved Neubauer rulings and an etched grid in the chamber. The ruling is the centerline of a group of three squares in which cells are counted.

A single drop of culture is applied in this counting method using a Pasteur pipette. The cell numbers in the given sample are counted directly in 10-20 high microscope fields. Based on the average number of cells per field, the number of bacteria per milliliter of the original sample can be obtained.
The final concentration of cells in the given sample is calculated using the formula:[3]
Total cells counted x 2.0×10 7 x dilution factor/ # small squares counted = cells/ml
The direct microscopic count is also done using fluorescent dyes. These dyes are popular in labs because of their ability to stain all bacterial species in a given sample, a particular species, or a specific component of cells. A few examples of fluorescent dyes are cyanoditolyl tetrazolium chloride (CTC), auramine, acridine orange, and rhodamine.
The most common fluorescent dye used to stain bacteria is acridine orange. In this method, a known sample volume is stained with acridine orange and then filtered through a 0.22 µm filter. The filter traps the bacteria that are then examined under the fluorescent microscope. By counting bacteria in a defined area of the filter, the concentration of bacteria in the original sample can be determined.
Bacteria enumeration is the process of determining the number of bacterial cells in a given sample. The counting of bacterial cells has four categories based on the purpose of the experiment: direct, indirect, viable, and total cell count. The categories are combined in four different ways to serve the experimental purpose in different techniques.
Many methods have been developed to count the numbers of bacteria in labs, but the most frequently used ones are standard plate count, turbidimetric method, and direct microscopic count.
The techniques are essential in food and beverage industries, where counting the numbers of bacteria in the given food or beverage sample is essential to learn if they are safe to consume and are not contaminated. Besides this, bacteria enumeration also has applications in agriculture and processing industries.
Despite making the enumeration process easier and smoother, the available techniques have several limitations. Thus offering young researchers the chance to develop techniques that cover the limitations and provide a faster approach for the enumeration process.
