

Genetic engineering is a technology in molecular biology that uses recombinant DNA technology to manipulate genetic materials. The technology aims to construct customized proteins or modify the working of biological systems, which leads to the creation of genetically modified organisms (GMOs).
The application of genetic engineering has improved and developed new techniques and technologies that advance our understanding of biological phenomena and elevate our well-being.[1-2]
However, there are a few concerns, such as ethical issues and the safety of genetically modified foods about the technology. This article discusses the benefits of genetic engineering alongside its application with the problems and risks associated with it.
Genetic engineering involves combining biochemistry and molecular biology techniques that start with the isolation of the genetic materials, followed by molecular cloning. It ends when GMOs, the organisms possessing recombinant DNA, are created.
Briefly, genetic engineering involves:[1]
The purpose of this process is to acquire genetic molecules from the organism of interest. Depending on the end-use, the genetic material can be DNA isolated from the organism of interest or ribonucleic acids (RNA) extracted from the tissues or cells of interest.
Isolated RNAs are reverse-transcribed to generate complementary DNA (cDNA) molecules. This strategy is useful when dealing with mRNAs of genes that need some form of post-transcriptional modifications for their expression.
This process involves cutting, assembling, amplifying, and introducing the recombinant DNA into the host organisms. It consists of the following steps:
This step serves to single out the region of interest by eliminating non-target DNA molecules from the target ones. Traditionally, DNA fragmentation uses restriction enzymes, also known as restriction endonucleases, to cut the DNA at specific sequences into fragments.
It may also involve using the polymerase chain reaction (PCR) technique to modify or edit the nucleotide sequences of the target region. PCR or the digested fragments are separated by gel electrophoresis and subsequently purified for the next step.
In this step, the prepared DNA fragments join with a vector, a DNA molecule serving as a DNA-importing vehicle. The success of this step requires that both ends of the purified fragments possess sequences recognizable by the same restriction enzymes that cut in the multiple cloning site of the vector.
The enzyme DNA ligase catalyzes the joining of DNA fragments to the digested vector, creating the recombinant DNA.
This step introduces the constructed recombinant DNA into cells of the host organism, allowing it to be taken up and expressed. Transformation can occur using physical transformation approaches such as heat-shock transformation and electroporation or chemical transformation.
Host cells possessing the recombinant DNA are selected based on the selectable marker gene contained in the vector.
After selection, the host organisms can be cultured under the selective condition to increase the number of cells containing the recombinant DNA. The induction of the recombinant DNA expression results in the recombinant proteins.
Genetic engineering technology has been a springboard for developing new techniques and technologies that bring about the advancement of science and improve human welfare. The following are some of the advantages of genetic engineering and examples of real-world applications:
Genetic engineering is not a stand-alone technology, but it has improved and enhanced existing laboratory techniques. DNA sequencing technology is one of the laboratory techniques that became more powerful when recombinant DNA technology was incorporated into its workflow.[2]
For instance:
Sanger sequencing is a chain-termination DNA sequencing technique that uses dideoxynucleotides to terminate the synthesizing DNA chains. At the start of the reaction, oligonucleotides anneal to the complementary DNA sequences before the chain synthesis and termination occur.
The priming of oligonucleotides limits the sequencing of DNA fragments to only those with partially known DNA sequences. With molecular cloning, the ligation of the target DNA fragment to vectors at the site where the DNA sequences are known can overcome this limitation.
By doing so, oligonucleotides can anneal to the complementary region in the vector, enabling the sequencing of the unknown DNA fragment joined to the vector.[2]
Large-scale DNA sequencing, also known as shotgun DNA sequencing, is a DNA sequencing technique that accommodates long DNA fragments.
For example, in the early phase of the Human Genome Project, DNA is randomly broken and cloned into a bacteriophage sequencing vector, M13. This results in a library of clones containing the recombinant DNA.
The clones are randomly selected and sequenced until no new identical recombinant DNA appears. The repeated clones are pooled and hybridized in the library to mark and eliminate clones containing DNA fragments identical to those already sequenced.
This way, only the clones processing different DNA fragments are sequenced, and the covered region can be expanded.[3]
Apart from enhancing existing laboratory techniques, GMOs such as bacterial cells expressing recombinant proteins and transgenic animals have given scientists the tools to dissect, analyze, and describe several biological phenomena. These phenomena include the underlying cause of genetic diseases and disease pathology.
For example:
Gaucher disease (GD) is an inherited genetic disorder most prevalent in the Ashkenazi Jewish population. In GD patients, glucocerebroside, a type of glycolipids in the lysosome, is excessively accumulated. This is because of deficiencies in glucocerebrosidase (EC 3.2.1.45), the enzyme that usually degrades it.
Molecular cloning and sequencing of the gene encoding glucocerebrosidase, the alleles, and the neighboring region have enabled researchers to understand the change in the enzyme activity and its relationship to clinical manifestations of the disease.[4]
The spike proteins of severe acute respiratory syndrome and the Middle East respiratory syndrome coronaviruses (SARS-CoV and MERS-CoV, respectively) play a role in the virus entry into host cells. This knowledge stemmed from experiments that used recombinant proteins containing variations of the spike proteins and potential host targets.
Biochemical and cell biology experiments using recombinant proteins revealed that spike proteins from SARS-CoV and MERS-CoV interact with specific host proteins and hijack their way into the host cells.[5-6]
When a similar virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), emerged at the end of 2019, cloning, sequencing, and PCR site-directed mutagenesis of the gene encoding its spike protein could identify the beneficial mutation that became fixed in the genome of the new virus.
SARS-CoV2 recombinant spike protein expression illustrated its structure, host target, and viral cell entry mechanism, providing insights into potential vaccination targets and treatment strategies.[7-9]
Neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s dementia, and Pick’s disease are associated with an accumulation of microtubule-associated protein tau in the mammalian nervous system.
The nature of their manifestations has made the disease’s mechanisms and tau dysfunctionality challenging to study. In this case, genetic engineering provides the answer to the challenge in the creation of transgenic animals.
Caenorhabditis elegans, Drosophila melanogaster, Xenopus oocytes, and mice have been genetically manipulated to express various levels and forms of tau.
Studies of changes in the nervous system, tau dynamics, and neurological disease development in these genetically modified (GM) animals improve the understanding of neurodegenerative diseases and bring us closer to early diagnostic approaches and therapeutic intervention.[10]
Not only can genetic engineering provide a better understanding of how biological systems work and respond, but it can also be a tool when the knowledge is used to create new technologies.
Fluorescent protein technology is an example of a new technology built by genetic engineering. It started by discovering the green and red fluorescent proteins (GFP and RFP, respectively) in jellyfish and sea corals.
When exposed to the lights of a suitable wavelength, RFP and GFP are chromophores that emit fluorescence without any additional enzyme or cofactor. Cloning and sequencing of the genes encoding GFP and RFP provide information on the three-dimensional structure of the proteins, unveiling the formation of chromophores and the emission of fluorescence.
The findings permit scientists to use genetic engineering to manipulate the genes, hence creating the enhanced version of the proteins and changing the color of the chromophores. To date, the color palette of existing fluorescent proteins ranges in the blue-cyan, cyan-green, green, yellow, orange, red, and far-red spectra.
The same information also provided the basis for developing bimolecular fluorescence complementation (BiFC) assay, enabling scientists to confirm a protein-protein interaction and where the interaction occurs in living cells.[11-12]
Genetic engineering can use insights into the cause of a disease, its pathology, and its development to create new therapeutic approaches. Examples of these are:
Therapeutic proteins are proteins used in replacement therapy to treat genetic disorders or certain medical conditions. Therapeutic proteins can be extracted from human or animal cells, semi-synthesized, or genetically engineered in host cells in the laboratory. Examples of therapeutic recombinant proteins are human growth hormone and glucocerebrosidase used in replacement therapy for dwarfism and Gaucher disease.[1,4]
Gene therapy refers to a therapeutic strategy that overrides the effect of a dysfunctional gene allele in patients. It does so by introducing the patient’s somatic cells to the functional allele of the gene or to the nucleic acids that modify the function of the defective allele.[1]
In ex vivo gene therapy, the functional allele is inserted into the patient’s cells, and the transfected cells are transplanted back into the patient. One method is to use a viral vector vehicle to carry the functional gene into the cell. Here, genetic engineering replaces the viral genes involved in viral replication, or the nonessential genes, with the target gene.
In doing so, the viral vector cannot be replicated using the host machinery, and the protein of the inserted allele is created. The Oxford-AstraZeneca, Sputnik V, Johnson and Johnson, and Convidecia COVID-19 vaccines are based on this method. Instead of a functional gene allele, a region from the viral spike protein is inserted into the vector and later translated as a target for antibody induction.[1,13]
Alternatively, in vivo gene therapy directly delivers the gene products such as mRNA or short-interfering RNA (siRNA) to the patient’s cells. This method starts with constructing a recombinant DNA that contains the cDNA of interest in a vector of choice.
The recombinant DNA is subsequently transcribed in vitro by RNA polymerase, purified, modified, and packaged for injection into the patient.
The Pfizer-BioNTech, Moderna, and CureVac mRNA-based vaccines against SARS-CoV2 are developed based on this approach. Similar to the viral vector COVID-19 vaccines, a region of the spike protein is translated and used to elicit an immune response in the vaccinated person.[1,13]
The manipulation of DNA from different species can combine desirable features in one transgenic organism. GMOs can be engineered to display a combination of customized characteristics that are otherwise not producible by other means.
Notable examples of such GMOs or their products are:
Taliglucerase alfa, marketed as Elelyso, is the first FDA-approved plant-based pharmaceutical drug. It is a recombinant human glucocerebrosidase intended for enzyme replacement therapy in Gaucher disease patients.
Carrot cells, which do not naturally possess glucocerebrosidase, were genetically manipulated to express the protein and signal peptides necessary for posttranslational modification.
The choice of a plant-based production system was based on the fact that most people afflicted with Gaucher disease follow a Kosher diet, which prohibits non-certified animals or animal by-products.[14]
Golden Rice is the first transgenic plant genetically engineered to contain the entire beta-carotene biosynthesis pathway. Beta-carotene is a vitamin A precursor naturally produced in the leaf but not in the edible endosperm.
Genes encoding phytoene synthase from daffodils, carotene desaturase 1 and 2 from the bacterium Erwiniauredovora were cloned and constructed, and transferred to be expressed in the rice endosperm.
As the name suggests, golden rice produces yellow grains that are high in beta-carotene. It is thought that when consumed, beta-carotene in the grains can supplement vitamin A in the consumers. Since its inception, golden rice has been used as a parental line for several rice breeding programs.[1]
Despite the pros of genetic engineering technology, its use remains debatable decades after the conception of the technology. The concerns over the use of genetic engineering revolve around the following issues:[1]
Genetic engineering uses recombinant DNA technology to create genetically modified organisms or GMOs. It is one of the most powerful technologies available in molecular biology. On the other hand, it’s also one of the most controversial. Nevertheless, the benefits of genetic engineering are so much more than creating new GMOs.
Its application also improves existing techniques, unravels complex biological processes and brings about new technologies and novel approaches. When used within the bounds of ethical and safety concerns, it brings about the technological advancement that can improve the quality of life.
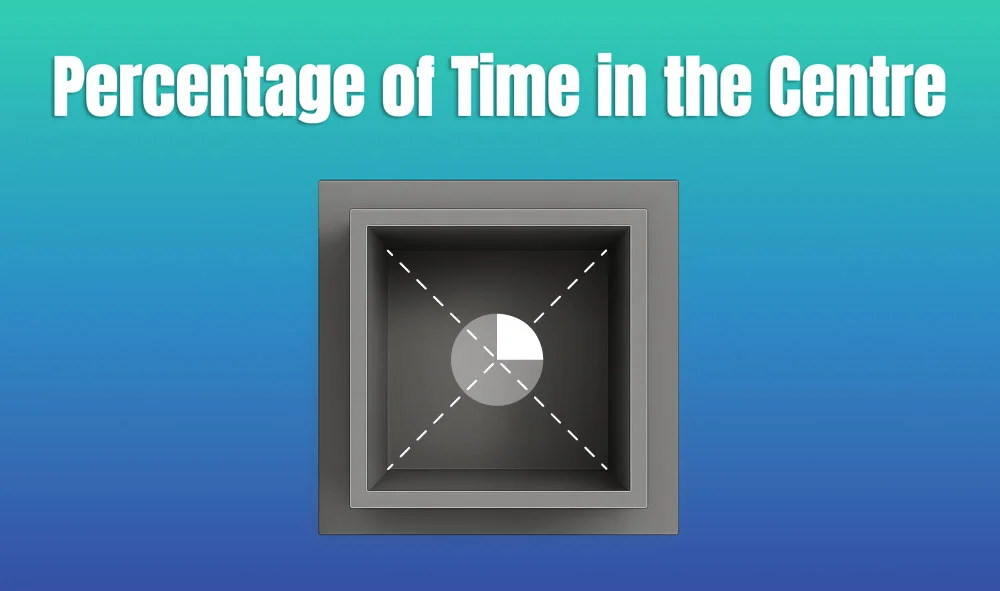
In behavioral neuroscience, the Open Field Test (OFT) remains one of the most widely used assays to evaluate rodent models of affect, cognition, and motivation. It provides a non-invasive framework for examining how animals respond to novelty, stress, and pharmacological or environmental manipulations. Among the test’s core metrics, the percentage of time spent in the center zone offers a uniquely normalized and sensitive measure of an animal’s emotional reactivity and willingness to engage with a potentially risky environment.
This metric is calculated as the proportion of time spent in the central area of the arena—typically the inner 25%—relative to the entire session duration. By normalizing this value, researchers gain a behaviorally informative variable that is resilient to fluctuations in session length or overall movement levels. This makes it especially valuable in comparative analyses, longitudinal monitoring, and cross-model validation.
Unlike raw center duration, which can be affected by trial design inconsistencies, the percentage-based measure enables clearer comparisons across animals, treatments, and conditions. It plays a key role in identifying trait anxiety, avoidance behavior, risk-taking tendencies, and environmental adaptation, making it indispensable in both basic and translational research contexts.
Whereas simple center duration provides absolute time, the percentage-based metric introduces greater interpretability and reproducibility, especially when comparing different animal models, treatment conditions, or experimental setups. It is particularly effective for quantifying avoidance behaviors, risk assessment strategies, and trait anxiety profiles in both acute and longitudinal designs.
This metric reflects the relative amount of time an animal chooses to spend in the open, exposed portion of the arena—typically defined as the inner 25% of a square or circular enclosure. Because rodents innately prefer the periphery (thigmotaxis), time in the center is inversely associated with anxiety-like behavior. As such, this percentage is considered a sensitive, normalized index of:
Critically, because this metric is normalized by session duration, it accommodates variability in activity levels or testing conditions. This makes it especially suitable for comparing across individuals, treatment groups, or timepoints in longitudinal studies.
A high percentage of center time indicates reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance. reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity, where animals exhibit persistent avoidance of the center due to heightened emotional reactivity. This metric can also distinguish between acute anxiety responses and enduring trait anxiety, especially in longitudinal or developmental studies. Its normalized nature makes it ideal for comparing across cohorts with variable locomotor profiles, helping researchers detect true affective changes rather than activity-based confounds.
Rodents that spend more time in the center zone typically exhibit broader and more flexible exploration strategies. This behavior reflects not only reduced anxiety but also cognitive engagement and environmental curiosity. High center percentage is associated with robust spatial learning, attentional scanning, and memory encoding functions, supported by coordinated activation in the prefrontal cortex, hippocampus, and basal forebrain. In contrast, reduced center engagement may signal spatial rigidity, attentional narrowing, or cognitive withdrawal, particularly in models of neurodegeneration or aging.
The open field test remains one of the most widely accepted platforms for testing anxiolytic and psychotropic drugs. The percentage of center time reliably increases following administration of anxiolytic agents such as benzodiazepines, SSRIs, and GABA-A receptor agonists. This metric serves as a sensitive and reproducible endpoint in preclinical dose-finding studies, mechanistic pharmacology, and compound screening pipelines. It also aids in differentiating true anxiolytic effects from sedation or motor suppression by integrating with other behavioral parameters like distance traveled and entry count (Prut & Belzung, 2003).
Sex-based differences in emotional regulation often manifest in open field behavior, with female rodents generally exhibiting higher variability in center zone metrics due to hormonal cycling. For example, estrogen has been shown to facilitate exploratory behavior and increase center occupancy, while progesterone and stress-induced corticosterone often reduce it. Studies involving gonadectomy, hormone replacement, or sex-specific genetic knockouts use this metric to quantify the impact of endocrine factors on anxiety and exploratory behavior. As such, it remains a vital tool for dissecting sex-dependent neurobehavioral dynamics.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity. Because it is normalized, this metric is especially helpful for distinguishing between genuine avoidance and low general activity.
Environmental Control: Uniformity in environmental conditions is essential. Lighting should be evenly diffused to avoid shadow bias, and noise should be minimized to prevent stress-induced variability. The arena must be cleaned between trials using odor-neutral solutions to eliminate scent trails or pheromone cues that may affect zone preference. Any variation in these conditions can introduce systematic bias in center zone behavior. Use consistent definitions of the center zone (commonly 25% of total area) to allow valid comparisons. Software-based segmentation enhances spatial precision.
Evaluating how center time evolves across the duration of a session—divided into early, middle, and late thirds—provides insight into behavioral transitions and adaptive responses. Animals may begin by avoiding the center, only to gradually increase center time as they habituate to the environment. Conversely, persistently low center time across the session can signal prolonged anxiety, fear generalization, or a trait-like avoidance phenotype.
To validate the significance of center time percentage, it should be examined alongside results from other anxiety-related tests such as the Elevated Plus Maze, Light-Dark Box, or Novelty Suppressed Feeding. Concordance across paradigms supports the reliability of center time as a trait marker, while discordance may indicate task-specific reactivity or behavioral dissociation.
When paired with high-resolution scoring of behavioral events such as rearing, grooming, defecation, or immobility, center time offers a richer view of the animal’s internal state. For example, an animal that spends substantial time in the center while grooming may be coping with mild stress, while another that remains immobile in the periphery may be experiencing more severe anxiety. Microstructure analysis aids in decoding the complexity behind spatial behavior.
Animals naturally vary in their exploratory style. By analyzing percentage of center time across subjects, researchers can identify behavioral subgroups—such as consistently bold individuals who frequently explore the center versus cautious animals that remain along the periphery. These classifications can be used to examine predictors of drug response, resilience to stress, or vulnerability to neuropsychiatric disorders.
In studies with large cohorts or multiple behavioral variables, machine learning techniques such as hierarchical clustering or principal component analysis can incorporate center time percentage to discover novel phenotypic groupings. These data-driven approaches help uncover latent dimensions of behavior that may not be visible through univariate analyses alone.
Total locomotion helps contextualize center time. Low percentage values in animals with minimal movement may reflect sedation or fatigue, while similar values in high-mobility subjects suggest deliberate avoidance. This metric helps distinguish emotional versus motor causes of low center engagement.
This measure indicates how often the animal initiates exploration of the center zone. When combined with percentage of time, it differentiates between frequent but brief visits (indicative of anxiety or impulsivity) versus fewer but sustained center engagements (suggesting comfort and behavioral confidence).
The delay before the first center entry reflects initial threat appraisal. Longer latencies may be associated with heightened fear or low motivation, while shorter latencies are typically linked to exploratory drive or low anxiety.
Time spent hugging the walls offers a spatial counterbalance to center metrics. High thigmotaxis and low center time jointly support an interpretation of strong avoidance behavior. This inverse relationship helps triangulate affective and motivational states.
By expressing center zone activity as a proportion of total trial time, researchers gain a metric that is resistant to session variability and more readily comparable across time, treatment, and model conditions. This normalized measure enhances reproducibility and statistical power, particularly in multi-cohort or cross-laboratory designs.
For experimental designs aimed at assessing anxiety, exploratory strategy, or affective state, the percentage of time spent in the center offers one of the most robust and interpretable measures available in the Open Field Test.
Written by researchers, for researchers — powered by Conduct Science.





Monday – Friday
9 AM – 5 PM EST
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.