

One of the most important pieces of equipment in the laboratory is the centrifuge, which facilitates the separation of samples of different densities. Centrifuge exists in different forms for different utility: gas centrifuge for isotope separation, human and non-human centrifuges for aeronautics and astronautics, industrial centrifuge separator as a coolant filtration system, geotechnical centrifuge for testing Geotechnical Engineering systems, and various centrifuges for commercial applications such as washing machines and sugar centrifugal machines.
The use of centrifuge systems started as early as mid-15th century when hand-driven centrifuge was used as a milk separator, and in 1864, Antonin Prandtl developed the first dairy centrifuge which separates the cream from milk. The potential of using the centrifuge system in the laboratory was exploited in 1869 by Friedrich Miescher. He was the first researcher to isolate nucleic acid from a cell with the use of a crude centrifuge system. The use of the centrifuge in the laboratory was then recognized and further developed which led to the development of a continuous centrifugal separator by Gustaf de Laval. The commercialization of centrifuge then surfaced as further improvements on the centrifuge system were made, such as Theodor Svedberg’s ultracentrifuge which enabled him to record sedimentation boundaries, Emile Henriot’s centrifuge which had very high rotational speeds, and many more.
Choosing the Best Benchtop Centrifuge
With a number of choices available in the market, it is necessary to know how to select the most appropriate and best type of centrifuge. Remember three significant factors while choosing a centrifuge.
Your requirements
It is essential to know your requirements before purchasing a centrifuge since there is a suitable and appropriate type of centrifuge available for each application. The most widely recognized applications that use centrifuge devices are medical and research laboratory tasks. The most commonly utilized centrifuges in these fields are micro-centrifuges, benchtop centrifuges, high-speed centrifuges, ultracentrifuges, and hematocrit centrifuges. For instance, ultracentrifuges are generally utilized to extract cellular components, for example, DNA, in the fields of molecular biology, biochemistry, and microbiology. Hematocrit centrifuge models, alternatively, are frequently utilized to divide blood components into the plasma and RBC layers.
Specifications
A few of the specifications that you ought to consider are:
The rate of centrifugation, generally known as revolutions per minute (RPM), is a term utilized by the developers to tell how quickly the centrifuge will rotate despite the size of the rotor. In contrast, the relative centrifugal force (RCF) is the force produced by the revolutions of the rotor and applied to the contents of the rotor which results in the separation of fluids in the centrifuge. The RPM and RCF forces are among the most important specifications to consider while selecting centrifuges because the requirement for the centrifugal force varies for the sedimentation of a different specimen. For instance, to accurately isolate specific bacterial cells from a specimen, high-speed or ultracentrifugation is required.
A few centrifuges have fixed speed, whereas other centrifuges have variable speed settings because of control panels or rotating frameworks that allow the user to choose a particular speed or power. This variable-speed option and rotary selection enable the users to utilize their device for a wide range of applications (multipurpose centrifuge).
The capacity of the device is determined by the maximum quantity or volume of samples that you can accommodate inside the rotor. Benchtop centrifuges, ultracentrifuges, or multipurpose centrifuges, can fit up to 1000 ml of sample per tube or bottle, based upon the model. The quantity of tubes or bottles these centrifuges can hold additionally varies depending on the model. The number and type of sample each centrifuge can hold relies upon the type of rotor, and in a few centrifuges, these rotors can be replaced with certain models that have up to 18 rotor options. In addition, a few centrifuges are assembled with refrigeration systems, which are helpful for temperature labile specimen.
To ensure that the centrifuge is easy to operate, try the various types of control panels and programs available on a centrifuge. It is important to opt for purchasing models with control panels that are user-friendly in case you are not familiar with controlling centrifuges so that you can accurately utilize the centrifuge. This will also enable the user to avoid making mistakes that could result in workplace accidents or disasters. A few centrifuges have additional features like alarms, safety locked covers, and automated rotor identification that could assist in the safety and comfort of carrying out centrifugation while performing multiple tasks with other laboratory tasks.
Total costs
It is important to keep in mind that a centrifuge with higher power will require a higher amount of energy to operate. Consequently, it is less energy-efficient as compared to the low-speed centrifuges. However, a few models are less expensive and could still deliver acceptable outcomes, although not as precise as expensive high-speed centrifuges. Another significant point to consider while choosing a centrifuge is that the device can be purchased with or without rotors; you need to purchase the rotors separately if the rotors are excluded from the model. In addition, the warranty of the device ought to be taken into consideration in case there is an unexpected turn of events that you did not anticipate.
These centrifuges, albeit having bigger dimensions than microcentrifuges, are still suitable to fit in your workspace for the compact design. The significant feature of these centrifuges is the significantly low operating noise level to avoid distractions when in use at your benchtop. Based on the parameters mentioned above, reviews of users, and nominations or awards won by the apparatus, here is our list that you can choose from:
Speed and performance – Variable speed; 200-4,000 RPM; maximum RCF of 20,913 x g
Instrument capacity –40 x 15 ml conical tubes; 56 x 13 mm/52 x 16 mm blood collection tubes; 4 x 250 ml buckets
Rotor options – 12 options
Ease of operation – Front panel with control knob and buttons
Extra feature/s – swing-out rotors; aerosol-tight; automatic stop
Power – 120V/60Hz
This centrifuge is best used for applications requiring large amounts or numbers of samples. It has a significantly high maximum RCF of 20,913 x g and a speed range of 200-4,000 RPM. This model can accommodate 250ml buckets, 15ml tubes, and 50ml tubes, with its four-place swing-out rotor and adapters. Hazardous samples can be used due to its aerosol-tight rotor and lid, and the centrifuge automatically stops when the lid is opened for safety. Its front panel has control buttons for quick and easy manipulation of settings or programs such as RPM, RCF and time.
Speed and performance – Variable speed; 200-6,000 RPM; maximum RCF of 4,180 x g
Instrument capacity – 0.2 ml to 50 ml tubes (depending on the rotor)
Rotor options – 4 options
Ease of operation – Front panel with control knob and buttons
Extra feature/s – can be operated with a swing-out rotor
Power – 120V/60Hz
This centrifuge can be used for both clinical and research applications. With four rotor options, it can accommodate various volumes of samples, from 0.2 ml up to 50 ml tubes. An example of its rotor options is the BS-Z206-0605 rotor, a swing-out rotor which is usually preferred by many clinical laboratories. Its maximum speed is 6,000 RPM, and the user can easily control its acceleration and deceleration with the control knob and buttons located at the front panel.
Speed and performance – Variable speed; 100-4,500 RPM; maximum RCF of 2,490 x g
Instrument capacity – 8 x 15 ml tubes; 12 x 3ml, 5ml, 7ml, or 10ml vacuum tubes with adapters
Rotor options – 1 option; angle rotor
Ease of operation – LCD panel, control knob, short-spin button, and timer
Extra feature/s – electrical lock and automatic release; advanced micro-computer
Power – 110V/60Hz
This clinical centrifuge is equipped with an angle rotor that can accommodate a range of volume of samples with the use of adapters. The advanced micro-computer of the model allows easy and complete control of RPM, RCF, and time, by displaying real-time parameters and symbols to show the centrifugation process. For safety purposes, it has an electrical lock with an automatic release when the rotor stops rotating.
Speed and performance – Variable speed; 550-3300 RPM; maximum RCF of 1350 x g
Instrument capacity – Eight: 15 mL tubes or 7 mL tubes
Rotor options – 1; fixed angle 45-degree polycarbonate
Ease of operation – Digital tachometer and controller
Extra feature/s – Brushless motor; manual lock
Power – 120V/60Hz
In contrast to the Ample Scientific Champion E-33 which has fixed speed, this model has variable speed settings. This model can accommodate eight samples, with volumes 15 mL or 7mL tubes. However, for 7mL tubes, you will need small tube sleeve inserts for it to fit inside the rotor, which is already included in the package when you buy this model.
Speed and performance – Fixed speed: 1000, 1500, 2000, or 3000 RPM; maximum RCF of 1,350 x g
Instrument capacity – 12 x 12ml tubes or 9ml vacuum tubes
Rotor options – 1; swing-out rotor
Ease of operation – front panel: LED display, control buttons, on/off switch
Extra feature/s – vibration-resistant design; automatic launch lock and stop
This low-speed benchtop centrifuge can be used in both clinical laboratories and medical schools with a capacity of twelve 12ml tubes or 9ml vacuum tubes (with the use of adapters). This model has a maintenance-free brushless motor, soft start and run down, an automatic launch when the lid is locked, and an automatic stop if the lid is opened for safety. The vibration-resistant design of C2204, it will surely not disturb the user when placed in the workplace.
Speed and performance – variable speed; 1000-4000 RPM (adjustable in 500 RPM increments); 1790 g RCF
Instrument capacity – Six tubes: 10 mL to 15mL
Rotor options – 1; fixed-angle rotor
Ease of operation – front panel: LED display, timer selection buttons, on/off button
Extra feature/s – continuous hold-spin function; last-spin memory; auto-snap; rubber suction feet
This model offers variable speed selection with the use of the front panel to set the settings. It has a timer that can be set up to 60 minutes, and a last-spin memory feature to help you minimize the time you spend on reconfiguring your settings. With the compact design, suctioned feet to keep it in place, and minimal noise during operation, this centrifuge is perfect for your workplace. Also, the auto-snap feature ensures safety which is enabled when the lid is opened before the cycle of the rotor is complete. The manufacturer offers a 1-year warranty.
Speed and performance – Variable speed; 1000-4000 RPM (adjustable in 100 RPM increments); 120 x g to 1933 x g RFC
Instrument capacity – twelve; 10 mL to 15 mL test tubes
Rotor options – 1; fixed-angle aluminum rotor
Ease of operation – front panel: LED display, timer selection buttons, on/off button
Extra feature/s – continuous hold-spin function; last-spin memory; electronic lock and safety release latch; rubber suction feet
This model offers higher RFC than the previous Premiere model and also provides low operating noise level of under 60 dB. It has a maximum instrument capacity of twelve samples of 10ml to 15ml test tubes. With the front panel, you can set your desired settings and not worry about having to set it again for another batch of samples (if the same settings should be applied) with its last-spin memory feature. Its electronic lock and safety release latch feature ensures your safety; the lock is triggered when the centrifuge starts spinning, and the latch allows you to open the unit if there’s an emergency. This machine also comes with a 1-year warranty.
Instruction Manual
Every centrifuge comes with a manual, and it includes information on cleaning, maintenance, and possibly lists of disinfectants that are safe to use. Manuals should be read beforehand and should be kept for future reference.
Cleaning
The materials used in the manufacturing of centrifuges vary; plastic, ceramic, aluminum, and stainless steel are most commonly employed. Refer to the manual for recommendations from the manufacturers on chemicals and equipment to use. Make sure to clean the centrifuge daily, or at least weekly, to ensure the longevity and durability of the item. Remove the rotor, container holders, and samples, before cleaning the interior of the centrifuge. Clean the interior bucket, specimen holder, rotary motors, and other parts of the centrifuge. Do not use caustic detergents or anything with chlorine as a disinfectant or sterilizer, nor should you use steel wool, wire brushes, and other abrasives, when cleaning the centrifuge. Use a sponge and water with mild detergent instead.
Remember to clean the exterior too. Never pour water directly, or flood the interior of the centrifuge with water. This will damage the sensors, wirings, and other parts of the centrifuge that are sensitive. After cleaning, make sure to dry each part and cavity of the centrifuge properly.
Samples and Spills
Do not centrifuge uncommon solvents or solutions without referring to the manual first. Handle the human blood samples with utmost care and precaution to avoid contamination.
The spilled sample on the rotor will be dispersed as a mist if the centrifuge is running. However, many rotors have sealed compartments that provide aerosol containment to detain the mist. Decontamination of the containers must be done immediately. If the model does not offer a sealed compartment, then the whole chamber must be disinfected.
Rotors
The forces exerted by the centrifugal field take a toll on the rotor. Do not attempt to run the rotor at speeds higher than its maximum. Refer to the manual for specific instructions for handling rotors and make sure to replace damaged rotors (if applicable) before using a centrifuge.
Tubes
During centrifugation, containers of the samples like glass tubes can break due to the force exerted by the system. Any fragments of the glass or spillage from the sample should be removed from the buckets, adapters, rubber liners, and rotor chamber before using the centrifuge again. Remember to do several dry runs without samples to ensure that there is no further damage, and clean the dust that might have come from sandblasting of the rotor chamber due to glass fragments between each run.
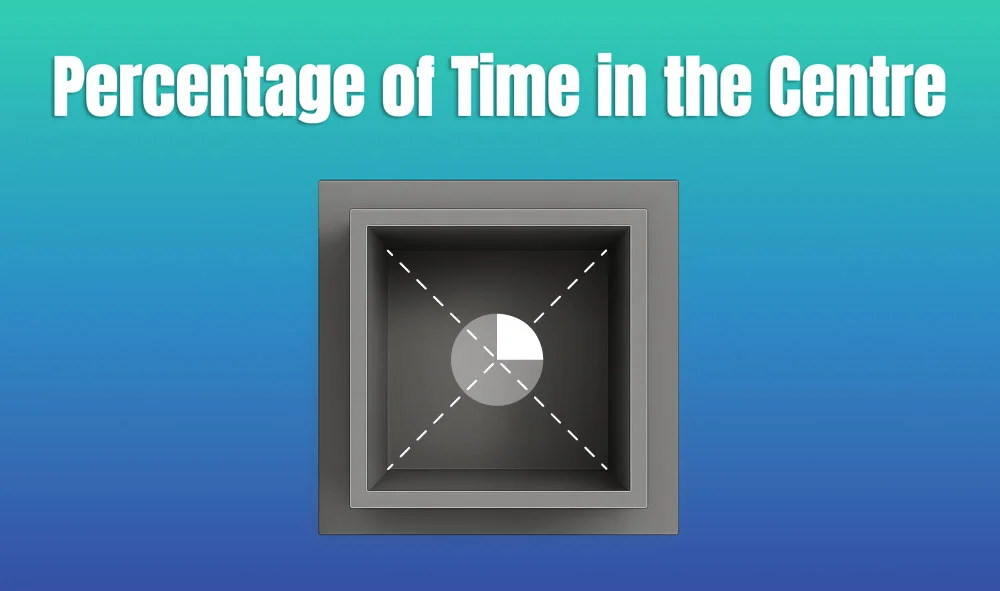
In behavioral neuroscience, the Open Field Test (OFT) remains one of the most widely used assays to evaluate rodent models of affect, cognition, and motivation. It provides a non-invasive framework for examining how animals respond to novelty, stress, and pharmacological or environmental manipulations. Among the test’s core metrics, the percentage of time spent in the center zone offers a uniquely normalized and sensitive measure of an animal’s emotional reactivity and willingness to engage with a potentially risky environment.
This metric is calculated as the proportion of time spent in the central area of the arena—typically the inner 25%—relative to the entire session duration. By normalizing this value, researchers gain a behaviorally informative variable that is resilient to fluctuations in session length or overall movement levels. This makes it especially valuable in comparative analyses, longitudinal monitoring, and cross-model validation.
Unlike raw center duration, which can be affected by trial design inconsistencies, the percentage-based measure enables clearer comparisons across animals, treatments, and conditions. It plays a key role in identifying trait anxiety, avoidance behavior, risk-taking tendencies, and environmental adaptation, making it indispensable in both basic and translational research contexts.
Whereas simple center duration provides absolute time, the percentage-based metric introduces greater interpretability and reproducibility, especially when comparing different animal models, treatment conditions, or experimental setups. It is particularly effective for quantifying avoidance behaviors, risk assessment strategies, and trait anxiety profiles in both acute and longitudinal designs.
This metric reflects the relative amount of time an animal chooses to spend in the open, exposed portion of the arena—typically defined as the inner 25% of a square or circular enclosure. Because rodents innately prefer the periphery (thigmotaxis), time in the center is inversely associated with anxiety-like behavior. As such, this percentage is considered a sensitive, normalized index of:
Critically, because this metric is normalized by session duration, it accommodates variability in activity levels or testing conditions. This makes it especially suitable for comparing across individuals, treatment groups, or timepoints in longitudinal studies.
A high percentage of center time indicates reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance. reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity, where animals exhibit persistent avoidance of the center due to heightened emotional reactivity. This metric can also distinguish between acute anxiety responses and enduring trait anxiety, especially in longitudinal or developmental studies. Its normalized nature makes it ideal for comparing across cohorts with variable locomotor profiles, helping researchers detect true affective changes rather than activity-based confounds.
Rodents that spend more time in the center zone typically exhibit broader and more flexible exploration strategies. This behavior reflects not only reduced anxiety but also cognitive engagement and environmental curiosity. High center percentage is associated with robust spatial learning, attentional scanning, and memory encoding functions, supported by coordinated activation in the prefrontal cortex, hippocampus, and basal forebrain. In contrast, reduced center engagement may signal spatial rigidity, attentional narrowing, or cognitive withdrawal, particularly in models of neurodegeneration or aging.
The open field test remains one of the most widely accepted platforms for testing anxiolytic and psychotropic drugs. The percentage of center time reliably increases following administration of anxiolytic agents such as benzodiazepines, SSRIs, and GABA-A receptor agonists. This metric serves as a sensitive and reproducible endpoint in preclinical dose-finding studies, mechanistic pharmacology, and compound screening pipelines. It also aids in differentiating true anxiolytic effects from sedation or motor suppression by integrating with other behavioral parameters like distance traveled and entry count (Prut & Belzung, 2003).
Sex-based differences in emotional regulation often manifest in open field behavior, with female rodents generally exhibiting higher variability in center zone metrics due to hormonal cycling. For example, estrogen has been shown to facilitate exploratory behavior and increase center occupancy, while progesterone and stress-induced corticosterone often reduce it. Studies involving gonadectomy, hormone replacement, or sex-specific genetic knockouts use this metric to quantify the impact of endocrine factors on anxiety and exploratory behavior. As such, it remains a vital tool for dissecting sex-dependent neurobehavioral dynamics.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity. Because it is normalized, this metric is especially helpful for distinguishing between genuine avoidance and low general activity.
Environmental Control: Uniformity in environmental conditions is essential. Lighting should be evenly diffused to avoid shadow bias, and noise should be minimized to prevent stress-induced variability. The arena must be cleaned between trials using odor-neutral solutions to eliminate scent trails or pheromone cues that may affect zone preference. Any variation in these conditions can introduce systematic bias in center zone behavior. Use consistent definitions of the center zone (commonly 25% of total area) to allow valid comparisons. Software-based segmentation enhances spatial precision.
Evaluating how center time evolves across the duration of a session—divided into early, middle, and late thirds—provides insight into behavioral transitions and adaptive responses. Animals may begin by avoiding the center, only to gradually increase center time as they habituate to the environment. Conversely, persistently low center time across the session can signal prolonged anxiety, fear generalization, or a trait-like avoidance phenotype.
To validate the significance of center time percentage, it should be examined alongside results from other anxiety-related tests such as the Elevated Plus Maze, Light-Dark Box, or Novelty Suppressed Feeding. Concordance across paradigms supports the reliability of center time as a trait marker, while discordance may indicate task-specific reactivity or behavioral dissociation.
When paired with high-resolution scoring of behavioral events such as rearing, grooming, defecation, or immobility, center time offers a richer view of the animal’s internal state. For example, an animal that spends substantial time in the center while grooming may be coping with mild stress, while another that remains immobile in the periphery may be experiencing more severe anxiety. Microstructure analysis aids in decoding the complexity behind spatial behavior.
Animals naturally vary in their exploratory style. By analyzing percentage of center time across subjects, researchers can identify behavioral subgroups—such as consistently bold individuals who frequently explore the center versus cautious animals that remain along the periphery. These classifications can be used to examine predictors of drug response, resilience to stress, or vulnerability to neuropsychiatric disorders.
In studies with large cohorts or multiple behavioral variables, machine learning techniques such as hierarchical clustering or principal component analysis can incorporate center time percentage to discover novel phenotypic groupings. These data-driven approaches help uncover latent dimensions of behavior that may not be visible through univariate analyses alone.
Total locomotion helps contextualize center time. Low percentage values in animals with minimal movement may reflect sedation or fatigue, while similar values in high-mobility subjects suggest deliberate avoidance. This metric helps distinguish emotional versus motor causes of low center engagement.
This measure indicates how often the animal initiates exploration of the center zone. When combined with percentage of time, it differentiates between frequent but brief visits (indicative of anxiety or impulsivity) versus fewer but sustained center engagements (suggesting comfort and behavioral confidence).
The delay before the first center entry reflects initial threat appraisal. Longer latencies may be associated with heightened fear or low motivation, while shorter latencies are typically linked to exploratory drive or low anxiety.
Time spent hugging the walls offers a spatial counterbalance to center metrics. High thigmotaxis and low center time jointly support an interpretation of strong avoidance behavior. This inverse relationship helps triangulate affective and motivational states.
By expressing center zone activity as a proportion of total trial time, researchers gain a metric that is resistant to session variability and more readily comparable across time, treatment, and model conditions. This normalized measure enhances reproducibility and statistical power, particularly in multi-cohort or cross-laboratory designs.
For experimental designs aimed at assessing anxiety, exploratory strategy, or affective state, the percentage of time spent in the center offers one of the most robust and interpretable measures available in the Open Field Test.
Written by researchers, for researchers — powered by Conduct Science.





Monday – Friday
9 AM – 5 PM EST
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.