

Colorimetry, as the name suggests, means the measurement of colors. In terms of chemical analysis, it is, more specifically, the measurement of the concentration of a particular compound (solute) in a colored solution (solvent). During scientific work, we often need to measure quantities of a particular compound in a mixture or the concentration of the solution. The trick is to identify the difference in colors of various mixtures and ascertain their absolute values. This is more informative and scientifically useful than simply having subjective judgments such as solutions being light or dark in color.[1]
Our eyes are not good enough to distinguish finer differences in colored solutions!
Light in the form of electromagnetic radiation enables a human eye to visualize objects. Visible light is measured in terms of wavelengths in the range of 400-700nm.[1][2] Scientists have identified that the human eyes have 3 different types of cone cells which helps perceive color. This phenomenon is called Trichromacy.[3] In order to create different colors, a human eye needs three different wavelengths of light; blue (short range), green (medium range), and red (long range).[4] There is a threshold beyond which the human eyes are not sensitive enough to distinguish small changes in the colors of a solution. Therefore, there is a need for a more sensitive measuring instrument which could give reliable and consistent results. This instrument is known as a Colorimeter. In determining the concentration of a solute in a solution, the Beer-Lambert law is used.
The first criterion for measuring the amount of solute in a given solvent is that the solution must be homogeneous. When a ray of light passes through the solution, a part of the light radiation is absorbed by the solution. The amount of light absorbed and transmitted is defined by the Beer-Lambert law.[5][6] This law is actually a combination of two different laws i.e., Beer’s law and Lambert’s law. Briefly, Beer’s law states that when a parallel beam of monochromatic light (i.e., having only one wavelength) passes through a solution, the amount of light absorbed by the solution is directly proportional to the amount of solute in the solution. Lambert’s law states that the absorbance of light by a colored solution depends on the length of the column and the volume of liquid through which light passes.
Beer and Lambert’s law draw links between light absorbed by the sample, the path it travels across the sample, and the concentration of the sample itself.
Mathematically, the equations of the Beer-lambert law can be described as follows; let’s assume that the ray of light of a particular wavelength has an intensity “Io” and after it has passed through the solution, the intensity is now “Ia”, while the solution has absorbed a portion of the intensity “Ib”. The following equation demonstrates the above phenomenon:
Io= Ia + Ib
The amount of light absorbed by the solution is directly proportional to the concentration of solute in the solution and the path length of the cuvette through which the light travels. This relationship between the absorption of light and the concentration of the solution is defined in Beer-Lambert’s law:
A= edC
Where,
A= Absorbance
e= Molar absorptivity (a measure that shows how well a solution absorbs light of a particular wavelength) (in mol L-1cm-1 )
d= Path length of the cuvette (in cm)
C= Concentration of the solute in solution (in mol L-1)
Once the light passes through the solution, it is collected into a detector. The relationship between the light that passed through the solution (I) and the original light applied on the sample (Io) is shown by the following formula:
T= I/ Io
Where, T= Transmittance (amount of light that passes through a solution)
The above two equations are represented in form of Absorbance (A) as follows:
A= -log10(I/ Io) = – log10 T
Absorbance is more commonly used than transmittance when determining the concentration of a solute in a solution.
The Colorimeter is the instrument used to ascertain the concentration of a solution by measuring the amount of absorbed light of a specific wavelength. One of the earliest and popular designs, Duboscq Colorimeter, invented by Jules Doboscq, dates back to the year 1870. A colorimeter has the following components; [7][8]
Ø A light source to illuminate the solution – usually a blue, green, and red LED.
Ø Filters for red, blue, and green wavelengths of light.
Ø A slit to focus beam of light.
Ø A condenser lens which focuses the beam of light into parallel rays.
Ø A cuvette to hold the solution. This is made either of glass, quartz, or plastic.
Ø A photoelectric cell, which is a vacuum filled cell used to measure the transmitted light and convert it into electrical output. These are made of light sensitive material such as selenium.
Ø An analog (e.g., a galvanometer) or digital meter to display output as transmittance or absorbance.
Having discussed the principles and components of a Colorimeter, it is time to look into the functionality of a colorimeter,[9] as follows;
A point to note is that the value obtained in the meter does not have any meaning since it is coming from a solution having an unknown concentration. We cannot translate that value into a concentration value because we do not have a pre-set reference. In order to identify the concentration of the solution, we need to quantify a standard of known concentrations. Standard solutions of various known concentrations are prepared and the absorbance is observed for each solution. A standard curve is drawn with these values, usually with absorbance on the Y axis and the concentration of the solution on the X axis. Using this method, the transmittance value for the solution can be used to extrapolate its concentration.
The spectrophotometer is the answer to that question. A spectrophotometer is like a colorimeter, in that it is also used in studying colored solutions, however, it is a bit more advanced. The Colorimeter, no doubt, is a fast, inexpensive way to quantitate solutes in a solution. However, like all techniques, this instrument also has some drawbacks,[9] as follows;
As technology progresses, so does the technical advancements in the field of colorimetry. There are now more sophisticated colorimeters than the Duboscq Colorimeter. Also, there now exists a smartphone app to do colorimetry, making the process more convenient and inexpensive.[18][19]
Colorimetry is a very quick and efficient way of analyzing colored solutions or any colored substance. Decades of research has enabled development of high end colorimeters which are more precise and convenient to use. The immense potential of colorimetry in terms of applications ranging from food, textiles, soil, and scientific research proves that this technique will not be getting redundant any time soon.
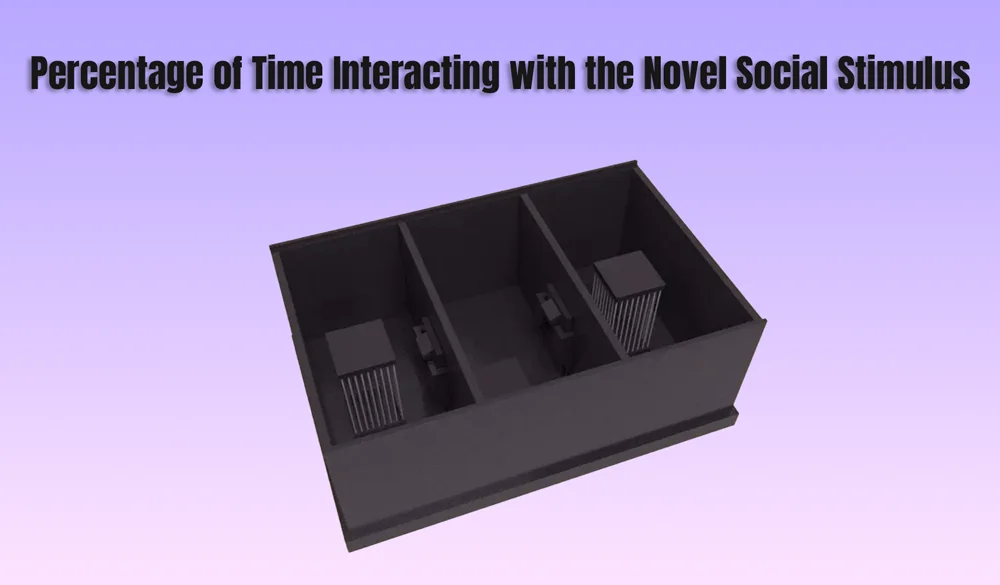
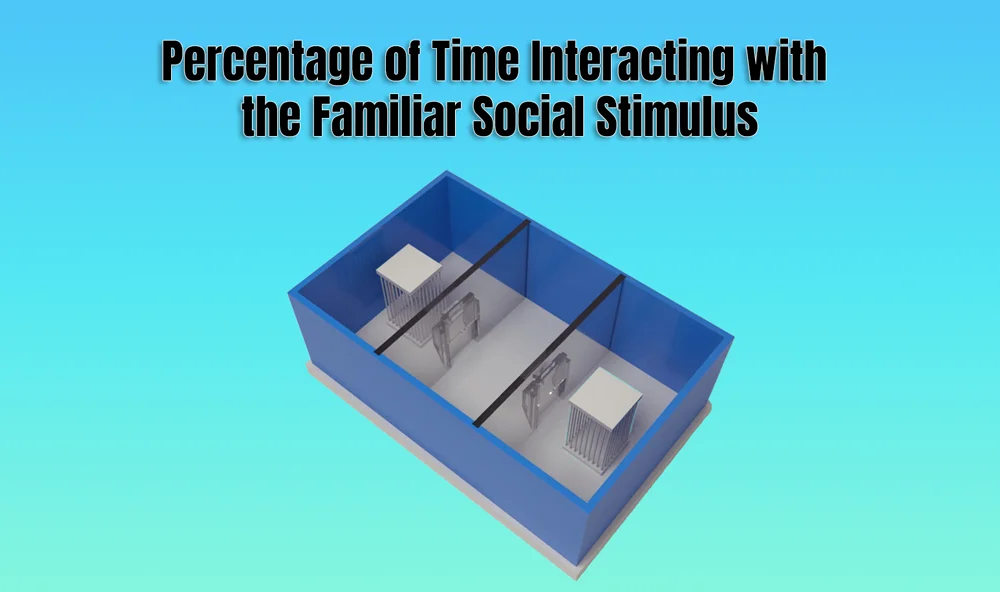
In behavioral neuroscience, the Open Field Test (OFT) remains one of the most widely used assays to evaluate rodent models of affect, cognition, and motivation. It provides a non-invasive framework for examining how animals respond to novelty, stress, and pharmacological or environmental manipulations. Among the test’s core metrics, the percentage of time spent in the center zone offers a uniquely normalized and sensitive measure of an animal’s emotional reactivity and willingness to engage with a potentially risky environment.
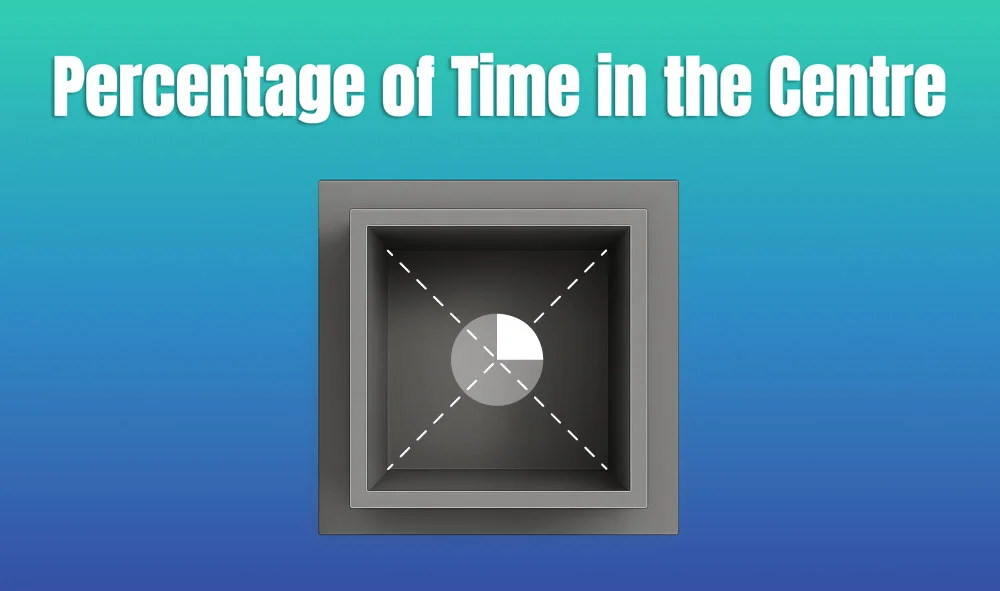
This metric is calculated as the proportion of time spent in the central area of the arena—typically the inner 25%—relative to the entire session duration. By normalizing this value, researchers gain a behaviorally informative variable that is resilient to fluctuations in session length or overall movement levels. This makes it especially valuable in comparative analyses, longitudinal monitoring, and cross-model validation.
Unlike raw center duration, which can be affected by trial design inconsistencies, the percentage-based measure enables clearer comparisons across animals, treatments, and conditions. It plays a key role in identifying trait anxiety, avoidance behavior, risk-taking tendencies, and environmental adaptation, making it indispensable in both basic and translational research contexts.
Whereas simple center duration provides absolute time, the percentage-based metric introduces greater interpretability and reproducibility, especially when comparing different animal models, treatment conditions, or experimental setups. It is particularly effective for quantifying avoidance behaviors, risk assessment strategies, and trait anxiety profiles in both acute and longitudinal designs.
This metric reflects the relative amount of time an animal chooses to spend in the open, exposed portion of the arena—typically defined as the inner 25% of a square or circular enclosure. Because rodents innately prefer the periphery (thigmotaxis), time in the center is inversely associated with anxiety-like behavior. As such, this percentage is considered a sensitive, normalized index of:
Critically, because this metric is normalized by session duration, it accommodates variability in activity levels or testing conditions. This makes it especially suitable for comparing across individuals, treatment groups, or timepoints in longitudinal studies.
A high percentage of center time indicates reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance. reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity, where animals exhibit persistent avoidance of the center due to heightened emotional reactivity. This metric can also distinguish between acute anxiety responses and enduring trait anxiety, especially in longitudinal or developmental studies. Its normalized nature makes it ideal for comparing across cohorts with variable locomotor profiles, helping researchers detect true affective changes rather than activity-based confounds.
Rodents that spend more time in the center zone typically exhibit broader and more flexible exploration strategies. This behavior reflects not only reduced anxiety but also cognitive engagement and environmental curiosity. High center percentage is associated with robust spatial learning, attentional scanning, and memory encoding functions, supported by coordinated activation in the prefrontal cortex, hippocampus, and basal forebrain. In contrast, reduced center engagement may signal spatial rigidity, attentional narrowing, or cognitive withdrawal, particularly in models of neurodegeneration or aging.
The open field test remains one of the most widely accepted platforms for testing anxiolytic and psychotropic drugs. The percentage of center time reliably increases following administration of anxiolytic agents such as benzodiazepines, SSRIs, and GABA-A receptor agonists. This metric serves as a sensitive and reproducible endpoint in preclinical dose-finding studies, mechanistic pharmacology, and compound screening pipelines. It also aids in differentiating true anxiolytic effects from sedation or motor suppression by integrating with other behavioral parameters like distance traveled and entry count (Prut & Belzung, 2003).
Sex-based differences in emotional regulation often manifest in open field behavior, with female rodents generally exhibiting higher variability in center zone metrics due to hormonal cycling. For example, estrogen has been shown to facilitate exploratory behavior and increase center occupancy, while progesterone and stress-induced corticosterone often reduce it. Studies involving gonadectomy, hormone replacement, or sex-specific genetic knockouts use this metric to quantify the impact of endocrine factors on anxiety and exploratory behavior. As such, it remains a vital tool for dissecting sex-dependent neurobehavioral dynamics.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity. Because it is normalized, this metric is especially helpful for distinguishing between genuine avoidance and low general activity.
Environmental Control: Uniformity in environmental conditions is essential. Lighting should be evenly diffused to avoid shadow bias, and noise should be minimized to prevent stress-induced variability. The arena must be cleaned between trials using odor-neutral solutions to eliminate scent trails or pheromone cues that may affect zone preference. Any variation in these conditions can introduce systematic bias in center zone behavior. Use consistent definitions of the center zone (commonly 25% of total area) to allow valid comparisons. Software-based segmentation enhances spatial precision.
Evaluating how center time evolves across the duration of a session—divided into early, middle, and late thirds—provides insight into behavioral transitions and adaptive responses. Animals may begin by avoiding the center, only to gradually increase center time as they habituate to the environment. Conversely, persistently low center time across the session can signal prolonged anxiety, fear generalization, or a trait-like avoidance phenotype.
To validate the significance of center time percentage, it should be examined alongside results from other anxiety-related tests such as the Elevated Plus Maze, Light-Dark Box, or Novelty Suppressed Feeding. Concordance across paradigms supports the reliability of center time as a trait marker, while discordance may indicate task-specific reactivity or behavioral dissociation.
When paired with high-resolution scoring of behavioral events such as rearing, grooming, defecation, or immobility, center time offers a richer view of the animal’s internal state. For example, an animal that spends substantial time in the center while grooming may be coping with mild stress, while another that remains immobile in the periphery may be experiencing more severe anxiety. Microstructure analysis aids in decoding the complexity behind spatial behavior.
Animals naturally vary in their exploratory style. By analyzing percentage of center time across subjects, researchers can identify behavioral subgroups—such as consistently bold individuals who frequently explore the center versus cautious animals that remain along the periphery. These classifications can be used to examine predictors of drug response, resilience to stress, or vulnerability to neuropsychiatric disorders.
In studies with large cohorts or multiple behavioral variables, machine learning techniques such as hierarchical clustering or principal component analysis can incorporate center time percentage to discover novel phenotypic groupings. These data-driven approaches help uncover latent dimensions of behavior that may not be visible through univariate analyses alone.
Total locomotion helps contextualize center time. Low percentage values in animals with minimal movement may reflect sedation or fatigue, while similar values in high-mobility subjects suggest deliberate avoidance. This metric helps distinguish emotional versus motor causes of low center engagement.
This measure indicates how often the animal initiates exploration of the center zone. When combined with percentage of time, it differentiates between frequent but brief visits (indicative of anxiety or impulsivity) versus fewer but sustained center engagements (suggesting comfort and behavioral confidence).
The delay before the first center entry reflects initial threat appraisal. Longer latencies may be associated with heightened fear or low motivation, while shorter latencies are typically linked to exploratory drive or low anxiety.
Time spent hugging the walls offers a spatial counterbalance to center metrics. High thigmotaxis and low center time jointly support an interpretation of strong avoidance behavior. This inverse relationship helps triangulate affective and motivational states.
By expressing center zone activity as a proportion of total trial time, researchers gain a metric that is resistant to session variability and more readily comparable across time, treatment, and model conditions. This normalized measure enhances reproducibility and statistical power, particularly in multi-cohort or cross-laboratory designs.
For experimental designs aimed at assessing anxiety, exploratory strategy, or affective state, the percentage of time spent in the center offers one of the most robust and interpretable measures available in the Open Field Test.
Written by researchers, for researchers — powered by Conduct Science.





Monday – Friday
9 AM – 5 PM EST
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.