$3,490.00
The Drosophila Visual and Olfactory Apparatus is designed to study sensorimotor interactions in Drosophila.
In this setup, flies are suspended between an optical sensor and an infrared LED. The movement of their wings casts a shadow onto the sensor. Electronic components monitor the motion of both wings, measuring the amplitude and frequency of each wing stroke.
While tethered, flies attempt to steer by adjusting the difference in amplitude between the left and right wingbeats (∆WBA). To simulate closed-loop conditions, the time-varying ∆WBA signal is linked to the angular velocity of a visual pattern created by a cylindrical array of green LEDs.
Mazeengineers provides the Drosophila Visual and Olfactory Apparatus for these studies.

MazeEngineers empowers preclinical neuroscience research with meticulously designed, customizable behavioral apparatuses. From manual classic mazes to fully automated smart systems, we provide the tools scientists need to capture high-quality, reproducible data for studies on learning, memory, anxiety, and depression.
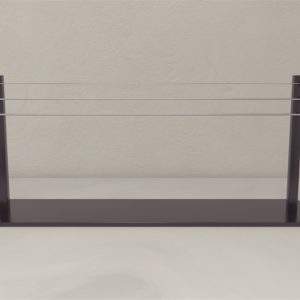


Features |
Black opaque device with a cylindrical flight arena |
Elevated platform with infrared light |
Sensor to analyze the wingbeats of the subjects |
Visual cues |
The bottom of the apparatus is provided with two 30 ml vials to carry both odor and odorless stimuli |
Delivery tubes |
The Drosophila Visual and Olfactory Apparatus is instrumental in studying sensorimotor interactions in Drosophila. Similar to other flying insects, Drosophila relies on multiple sensory modalities and precise motor control for survival and environmental interaction (Trimarchi & Schneiderman, 1995; Brembs & Heisenberg, 2001). They integrate various sensory inputs and relay this information to central pattern generators to execute motor functions (Fyre & Dickinson, 2004).
This apparatus, widely used for assessing visual and olfactory sensorimotor interactions, was originally created by Götz in 1964 and subsequently enhanced by Heisenberg and Wolf in 1984. It features a cylindrical flight arena where subjects are statically tethered. Visual stimuli, such as vertical stripe patterns expanding from the right and contracting to the left, are provided via wrap-around LEDs. Olfactory cues are delivered through vials with a mass flow controller at the bottom of the apparatus. The setup uses infrared light from above to cast the subject’s wingbeat shadow on a sensor at the bottom, enabling measurement of motor responses to visual and olfactory cues, either independently or combined.
The Drosophila Visual and Olfactory Apparatus is highly effective in exploring complex sensory-motor interactions, crucial for understanding navigation and foraging behaviors in Drosophila.
Other tools used to study Drosophila behaviors include the Drosophila Gravitaxis Maze, the Drosophila Maze Array, the Drosophila Y Maze, and the Drosophila Shallow Chamber.
The Drosophila Visual and Olfactory Apparatus is a black, opaque device featuring a cylindrical flight arena. An elevated platform with infrared lighting is positioned above the arena. The apparatus includes a sturdy support system that statically tethers the subject in the center of the cylindrical arena. At the bottom, a sensor is installed to analyze the subjects’ wingbeats. The inner wall of the cylindrical arena is lined with green LEDs that provide visual cues to the subject.
The wingbeat sensor’s output is linked to the LEDs, creating a closed-loop visual environment. At the base, two 30 ml vials contain either odor or odorless stimuli. These vials are connected to delivery tubes that lead to the distal end of a single pipette tip (4 mm long and less than 1 mm³ in volume). A continuous stream of vapors is maintained at a speed of 280 mm/s, facilitated by a mass flow air controller connected to the vials through a solenoid valve. This valve automatically alternates vapor delivery between odor and odorless cues. Additionally, a brass suction tube is situated behind the subject to remove any residual odor cues.
Maintain subjects on a 12-hour light/12-hour dark cycle and perform experiments within 6 hours of the start of the experimental day. Ensure the apparatus is cleaned and ventilated before each trial. Preload the delivery tubes with odor vapors from the vials to prevent delays in vapor delivery caused by the solenoid switch. Tether each subject at the dorsal junction of the head and thorax to a small tungsten wire under cold anesthesia, and allow at least 1 hour for recovery before starting the experiment. Use a tracking and recording system like Noldus Ethovision XT to assist with observations.
Tether the subject in the center of the cylindrical tube between the wingbeat sensor and the infrared diode. Present the visual stimulus through green LEDs on the inner lining of the cylindrical arena. Provide an odorless cue from a vial at the bottom of the apparatus. Introduce a collision stimulus by creating a 1.25-second bias in the loop. Conduct the trial for 30 seconds.
Tether the subject in the center of the cylindrical tube between the wingbeat sensor and the infrared diode. Present the visual stimulus through green LEDs on the inner lining of the cylindrical arena. Provide an odor cue from a vial at the bottom of the apparatus. Conduct the trial for 30 seconds.
Conduct the experiment with one vial containing an odor cue and the other an odorless cue. Tether the subject in the center of the cylindrical tube between the wingbeat sensor and the infrared diode. Present the visual stimulus through green LEDs. Introduce a collision stimulus by creating a 1.25-second bias in the closed-loop. Conduct the trial for 30 seconds.
Frye and Dickinson (2003) explored how visual and olfactory stimuli interact to influence wing motor control during flight in Drosophila melanogaster. They used 2-3 day-old female flies (n=22) tethered in the Drosophila Visual and Olfactory Apparatus to test motor responses to these stimuli. Vinegar served as the olfactory stimulus, while a LED-generated vertical stripe expanding left and contracting right was used as the visual stimulus. Flies were exposed to the stimuli individually and in combination. A collision stimulus was introduced by adding a 2.5-second bias to the closed-loop.
The findings demonstrated that flies adjusted their wingbeat frequency and amplitude in response to both visual and olfactory stimuli, whether presented separately or together. During tethered flight in closed-loop conditions, the flies (n=16) showed a symmetrical increase in wingbeat frequency and amplitude when exposed to the olfactory stimulus. However, no change in the magnitude or timing of collision avoidance reflexes was observed under closed-loop conditions (n=10).
Under visual closed-loop conditions, flies (n=10) reacted to lateral expansion with asymmetric changes in wingbeat amplitude, leading to a reduction in total wingbeat amplitude. The presence of an odor stimulus did not alter the magnitude or duration of visual reflexes (n=16). A linear interaction was noted between motor responses to visual and olfactory stimuli, where the combined response was approximately the sum of the individual responses to each stimulus (n=22).
The study concluded that visual and olfactory stimuli elicit independent motor responses that overlay when presented together. It was observed that during flight, Drosophila navigates using olfactory cues, while visual stimuli enhance olfactory-driven motor responses by ceasing or resetting them.
The Drosophila Visual and Olfactory Apparatus is user-friendly and does not necessitate extensive pretraining of subjects. Task performance is straightforward and can be completed in a short amount of time. This apparatus allows for the introduction of various visual stimuli.
The Drosophila Visual and Olfactory Apparatus is restricted to odor and visual cues. Task performance can be affected by improper handling and the presence of unsolicited stimuli. Additionally, variables such as the age, gender, and strain of the subjects may influence their performance in the tasks.
| Color | black |
|---|---|
| Species | Drosophila |
| Display Type | LED |
| arena_type | cylindrical flight arena |
| led_color | green |
| vial_capacity | 30 ml |
| pipette_tip_length | 4 mm |
| pipette_tip_volume | less than 1 mm³ |
| vapor_stream_speed | 280 mm/s |
| collision_stimulus_duration | 1.25 seconds |
| trial_duration | 30 seconds |
| lighting_type | infrared |
| tethering_material | tungsten wire |
| recovery_time | at least 1 hour |
| experimental_window | within 6 hours of start of experimental day |
| light_cycle | 12-hour light/12-hour dark cycle |