
The US is the largest market for medical devices, worth approximately $156 billion (as of 2017), and is expected to reach $208 billion by 2023. Given these numbers, it’s no surprise that high-risk medical devices must undergo strict regulatory and approval processes. One of them is the pre-market approval (PMA) for medical devices.
The pre-market approval for high-risk medical devices guarantees that medical devices are safe and effective at the same time. This pathway also ensures that high-risk devices can reach the public adequately in order to improve health outcomes and save lives.
The medical device market is expanding and improving constantly. Medical device regulations have also changed significantly over the last decades. The pre-market approval for medical devices gained popularity in the 80s after the notorious intrauterine contraceptive device Dalkon Shield was found to lead to inflammation, miscarriage, and fatalities (Rome et al., 2014).
To be more precise, in 1976, new amendments to the Food, Drug, and Cosmetic Act of 1938 widened the role of the US Food and Drug Administration (FDA) in medical device testing, with the Center for Devices and Radiological Health being the main body responsible for medical device review.
Similar to the strict FDA drug regulations, the 1976 Medical Device Amendments now enforces medical device manufacturers to follow strict testing, marketing, and surveillance procedures. It is worth noting that designing and marketing a medical device can take from three to seven years, as opposed to the burdensome process of drug approval which can last 10-15 years (Purnama & Drago, 2019).
When a new medical device is required as a solution to a health problem, this device undergoes a cycle of research, animal testing, preclinical trials, and redesign. After that, the device has to be classified, approved through a specific pathway, and registered by the FDA.
Depending on its classification, any novel device can go through one of the following regulatory pathways: pre-marketing notification, pre-market approval, or the humanitarian device exemption. We should note that Class I devices (e.g., bandages) do not fall under any pre-market notification or quality system requirements. On the other hand, Class II devices (e.g., powered wheelchairs) require pre-marketing notification or a 510(k) submission.
The pre-market approval process of Class III devices is the most rigorous pathway. It requires in-depth clinical evaluations on safety and effectiveness before manufacturers can register high-risk devices. Interestingly, the humanitarian device exemption also requires devices to be safe but does not require data on effectiveness. Note this pathway encourages the design of devices for rare conditions – in fewer than 4,000 people per year.
Here we should note that post-market data and post-approval surveillance are also needed to ensure high effectiveness and safety for some high-risk devices. For instance, 522 post-market surveillance studies may be required for life-supporting devices implanted for more than a year (Rome et al., 2014).
The pre-market approval is the most stringent regulatory pathway for medical devices in the US. As stated above, it’s used to guarantee the safety and effectiveness of Class III devices. The pre-market approval application requires detailed clinical information and should be scientifically sound.
It’s worth noting that the technical sections are crucial for a device to be approved. These sections can be divided into non-clinical lab studies (e.g., immunology, animal tests, shelf life) and clinical investigations (e.g., study protocols, device failures, patient information, statistical analysis). Like any other study report, applications should be well-organized and in compliance with recommended practices and industry standards
When it comes to high-risk devices, we should note that such medical devices are widely used in cardiac care. Implantable cardiac monitors, for example, are among the medical devices that must undergo the pre-market approval process to evaluate their safety and effectiveness.
We should note that implantable cardiac monitors are defined as electrophysiology devices used to detect electrical activity and arrhythmias. Such devices are inserted under the skin and are used for long-term monitoring.
Alarmingly, heart disease is the leading cause of death in the US. As both cardiologists and patients should be aware of the data that supports the use of such devices, regulatory bodies must ensure high transparency regarding risks and effectiveness. For instance, electromagnetic compatibility around other electronic devices should be stated clearly. When it comes to improvements to a medical device, data on comparative effectiveness is also crucial.
Another global health problem is diabetes, defined as the fastest growing chronic condition. Alarmingly, the number of patients with diabetes is rising; 422 million patients were registered in 2014, and it’s expected that these numbers will double by 2040 (Zhang et al., 2019).
Insulin pump therapy or continuous subcutaneous insulin infusion is one of the most important treatments for people with diabetes Type I, as well as Type II. In fact, this type of treatment shows positive glycogenic management and clinical outcomes.
While some insulin pumps are classified as Class II devices, those parts of an integrated system is Class III devices. Thus, these devices have to undergo strict pre-market approval to prevent risks, such as pump failure and insulin infusion blockage (Heinemann et al., 2014). Interestingly, data shows that the US FDA approved 2005 2018, 15 Class III devices for diabetes treatment.
Given the fact that the integration of science and technology is fundamental in foreign policy, it’s no surprise that the US is one of the countries that are willing to develop evidence-based policies and develop connections with other states seeking scientific progress. To provide an example, in 2015, the State Department established an Innovation Forum to connect with partners from Silicon Valley and other innovation centers (Turekian & Kishi, 2007).
Scientific methods are rapidly integrated into foreign affairs decision-making, while bilateral and multilateral meetings are regularly organized to tackle international concerns. To support governments around the world, not only in the US, scientists should be encouraged to work with each other to expand the world of scientific progress and tech innovation.
While the pre-market approval of medical devices guarantees a device’s safety and effectiveness, not many have been approved specifically in pediatric settings. Here we should note that the 2007 Pediatric Medical Device Safety and Improvement Act aims to stimulate pediatric medical device development.
Yet, figures show that most medical devices haven’t been studied in patients under 18 years of age. Hwang et al. (2014), for instance, identified 22 devices approved between 2008 and 2011 for use in children via the pre-market approval process and three via the humanitarian device exemption pathway. However, 88% had minimum approval ages of 18-21 years.
While pre-market approval is crucial to ensuring a device’s safety and effectiveness, practitioners should be aware of the process. For instance, many medical devices have been approved as supplements to existing devices without clearly stated improved effectiveness data (Rome et al., 2014). Note that this pre-market approval supplement pathway may be used to approve evolving technologies supported by limited data, so health practitioners should be highly cautious.
Patients should also be aware of potential dangers, as well as their legal rights. Note that the 2008 Riegel vs. Medtronic ruling makes it difficult for patients to seek damages from manufacturers of high-risk devices. Here we should give some more information about this ruling; after a balloon catheter caused damage during an angioplasty procedure, the Riegels sued the catheter’s manufacturer, Medtronic, but the Court dismissed the case.
When it comes to regulations, standards should be harmonized across the world at both- pre and post-marketing stages. Therefore, international databases and annual reports are needed to improve interoperability and boost scientific progress.
Medical devices are essential to ensure patient safety and health. There are different types of devices used to diagnose, treat, and monitor patients. In the US, in particular, medical devices are classified as Class I, Class II, and Class III devices according to their risk levels.
Class III devices are defined as high-risk devices and are deeply integrated into cardiac care and diabetes treatments. As such, they have to undergo strict regulatory and testing processes before they get approved. The pre-market approval process is the most rigorous pathway used to ensure that Class III devices are safe and effective.
Both patients and health practitioners should be aware of the regulatory pathways used to approve new medical devices. When it comes to pediatric care, for instance, the development of high-risk medical devices requires additional consideration and care.
Although the US is the largest market for medical devices – with the pre-market approval process being crucial to ensuring a high-risk medical device safety and effectiveness – international collaboration and transparent data are needed to support interoperability, scientific progress, and human health.
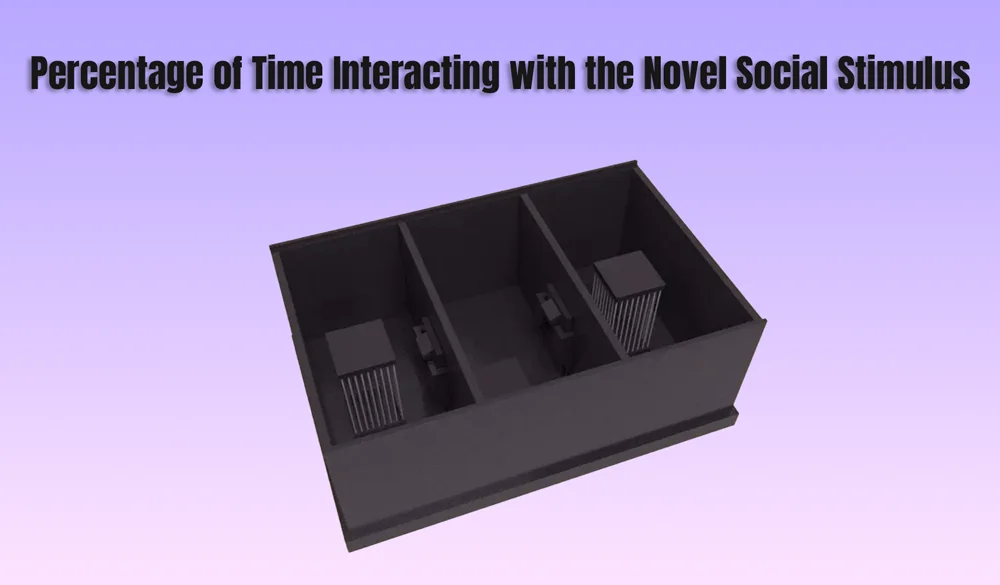
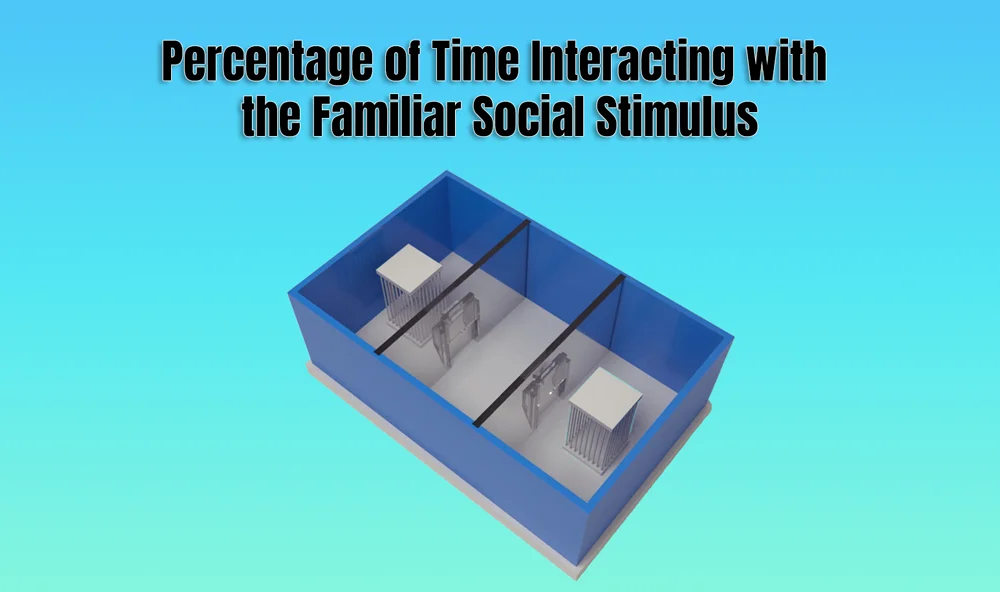
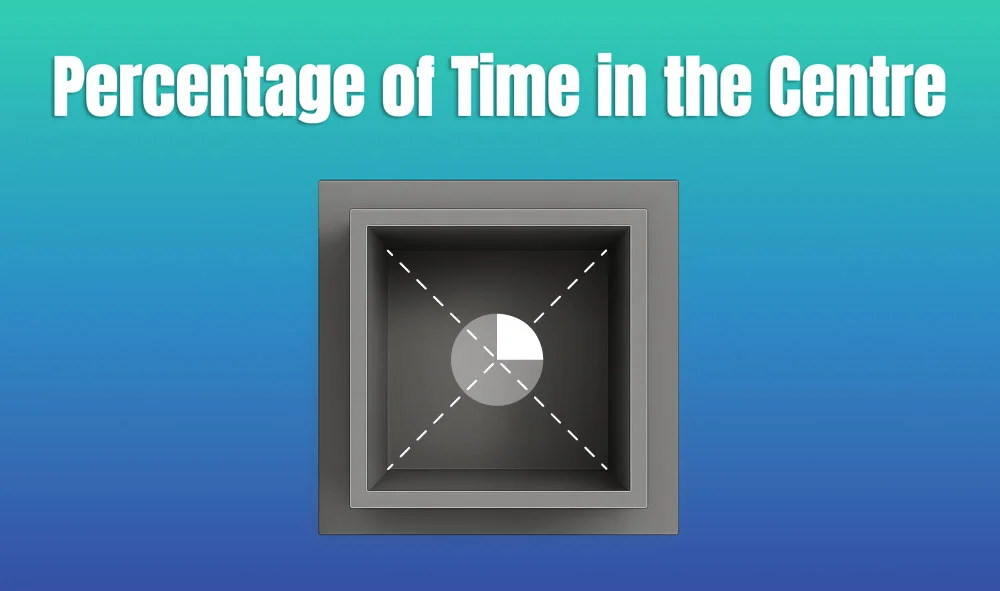
In behavioral neuroscience, the Open Field Test (OFT) remains one of the most widely used assays to evaluate rodent models of affect, cognition, and motivation. It provides a non-invasive framework for examining how animals respond to novelty, stress, and pharmacological or environmental manipulations. Among the test’s core metrics, the percentage of time spent in the center zone offers a uniquely normalized and sensitive measure of an animal’s emotional reactivity and willingness to engage with a potentially risky environment.
This metric is calculated as the proportion of time spent in the central area of the arena—typically the inner 25%—relative to the entire session duration. By normalizing this value, researchers gain a behaviorally informative variable that is resilient to fluctuations in session length or overall movement levels. This makes it especially valuable in comparative analyses, longitudinal monitoring, and cross-model validation.
Unlike raw center duration, which can be affected by trial design inconsistencies, the percentage-based measure enables clearer comparisons across animals, treatments, and conditions. It plays a key role in identifying trait anxiety, avoidance behavior, risk-taking tendencies, and environmental adaptation, making it indispensable in both basic and translational research contexts.
Whereas simple center duration provides absolute time, the percentage-based metric introduces greater interpretability and reproducibility, especially when comparing different animal models, treatment conditions, or experimental setups. It is particularly effective for quantifying avoidance behaviors, risk assessment strategies, and trait anxiety profiles in both acute and longitudinal designs.
This metric reflects the relative amount of time an animal chooses to spend in the open, exposed portion of the arena—typically defined as the inner 25% of a square or circular enclosure. Because rodents innately prefer the periphery (thigmotaxis), time in the center is inversely associated with anxiety-like behavior. As such, this percentage is considered a sensitive, normalized index of:
Critically, because this metric is normalized by session duration, it accommodates variability in activity levels or testing conditions. This makes it especially suitable for comparing across individuals, treatment groups, or timepoints in longitudinal studies.
A high percentage of center time indicates reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance. reduced anxiety, increased novelty-seeking, or pharmacological modulation (e.g., anxiolysis). Conversely, a low percentage suggests emotional inhibition, behavioral avoidance, or contextual hypervigilance.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity, where animals exhibit persistent avoidance of the center due to heightened emotional reactivity. This metric can also distinguish between acute anxiety responses and enduring trait anxiety, especially in longitudinal or developmental studies. Its normalized nature makes it ideal for comparing across cohorts with variable locomotor profiles, helping researchers detect true affective changes rather than activity-based confounds.
Rodents that spend more time in the center zone typically exhibit broader and more flexible exploration strategies. This behavior reflects not only reduced anxiety but also cognitive engagement and environmental curiosity. High center percentage is associated with robust spatial learning, attentional scanning, and memory encoding functions, supported by coordinated activation in the prefrontal cortex, hippocampus, and basal forebrain. In contrast, reduced center engagement may signal spatial rigidity, attentional narrowing, or cognitive withdrawal, particularly in models of neurodegeneration or aging.
The open field test remains one of the most widely accepted platforms for testing anxiolytic and psychotropic drugs. The percentage of center time reliably increases following administration of anxiolytic agents such as benzodiazepines, SSRIs, and GABA-A receptor agonists. This metric serves as a sensitive and reproducible endpoint in preclinical dose-finding studies, mechanistic pharmacology, and compound screening pipelines. It also aids in differentiating true anxiolytic effects from sedation or motor suppression by integrating with other behavioral parameters like distance traveled and entry count (Prut & Belzung, 2003).
Sex-based differences in emotional regulation often manifest in open field behavior, with female rodents generally exhibiting higher variability in center zone metrics due to hormonal cycling. For example, estrogen has been shown to facilitate exploratory behavior and increase center occupancy, while progesterone and stress-induced corticosterone often reduce it. Studies involving gonadectomy, hormone replacement, or sex-specific genetic knockouts use this metric to quantify the impact of endocrine factors on anxiety and exploratory behavior. As such, it remains a vital tool for dissecting sex-dependent neurobehavioral dynamics.
The percentage of center time is one of the most direct, unconditioned readouts of anxiety-like behavior in rodents. It is frequently reduced in models of PTSD, chronic stress, or early-life adversity. Because it is normalized, this metric is especially helpful for distinguishing between genuine avoidance and low general activity.
Environmental Control: Uniformity in environmental conditions is essential. Lighting should be evenly diffused to avoid shadow bias, and noise should be minimized to prevent stress-induced variability. The arena must be cleaned between trials using odor-neutral solutions to eliminate scent trails or pheromone cues that may affect zone preference. Any variation in these conditions can introduce systematic bias in center zone behavior. Use consistent definitions of the center zone (commonly 25% of total area) to allow valid comparisons. Software-based segmentation enhances spatial precision.
Evaluating how center time evolves across the duration of a session—divided into early, middle, and late thirds—provides insight into behavioral transitions and adaptive responses. Animals may begin by avoiding the center, only to gradually increase center time as they habituate to the environment. Conversely, persistently low center time across the session can signal prolonged anxiety, fear generalization, or a trait-like avoidance phenotype.
To validate the significance of center time percentage, it should be examined alongside results from other anxiety-related tests such as the Elevated Plus Maze, Light-Dark Box, or Novelty Suppressed Feeding. Concordance across paradigms supports the reliability of center time as a trait marker, while discordance may indicate task-specific reactivity or behavioral dissociation.
When paired with high-resolution scoring of behavioral events such as rearing, grooming, defecation, or immobility, center time offers a richer view of the animal’s internal state. For example, an animal that spends substantial time in the center while grooming may be coping with mild stress, while another that remains immobile in the periphery may be experiencing more severe anxiety. Microstructure analysis aids in decoding the complexity behind spatial behavior.
Animals naturally vary in their exploratory style. By analyzing percentage of center time across subjects, researchers can identify behavioral subgroups—such as consistently bold individuals who frequently explore the center versus cautious animals that remain along the periphery. These classifications can be used to examine predictors of drug response, resilience to stress, or vulnerability to neuropsychiatric disorders.
In studies with large cohorts or multiple behavioral variables, machine learning techniques such as hierarchical clustering or principal component analysis can incorporate center time percentage to discover novel phenotypic groupings. These data-driven approaches help uncover latent dimensions of behavior that may not be visible through univariate analyses alone.
Total locomotion helps contextualize center time. Low percentage values in animals with minimal movement may reflect sedation or fatigue, while similar values in high-mobility subjects suggest deliberate avoidance. This metric helps distinguish emotional versus motor causes of low center engagement.
This measure indicates how often the animal initiates exploration of the center zone. When combined with percentage of time, it differentiates between frequent but brief visits (indicative of anxiety or impulsivity) versus fewer but sustained center engagements (suggesting comfort and behavioral confidence).
The delay before the first center entry reflects initial threat appraisal. Longer latencies may be associated with heightened fear or low motivation, while shorter latencies are typically linked to exploratory drive or low anxiety.
Time spent hugging the walls offers a spatial counterbalance to center metrics. High thigmotaxis and low center time jointly support an interpretation of strong avoidance behavior. This inverse relationship helps triangulate affective and motivational states.
By expressing center zone activity as a proportion of total trial time, researchers gain a metric that is resistant to session variability and more readily comparable across time, treatment, and model conditions. This normalized measure enhances reproducibility and statistical power, particularly in multi-cohort or cross-laboratory designs.
For experimental designs aimed at assessing anxiety, exploratory strategy, or affective state, the percentage of time spent in the center offers one of the most robust and interpretable measures available in the Open Field Test.
Written by researchers, for researchers — powered by Conduct Science.





Monday – Friday
9 AM – 5 PM EST
DISCLAIMER: ConductScience and affiliate products are NOT designed for human consumption, testing, or clinical utilization. They are designed for pre-clinical utilization only. Customers purchasing apparatus for the purposes of scientific research or veterinary care affirm adherence to applicable regulatory bodies for the country in which their research or care is conducted.