

Take advantage of Neuralynx, Ethovision Integration, SMS and Email integration with the Conductor Science Software. No I/O Boxes Required
Learn More
This software provides an UI application for user to upload Tail Flick test data to PC.
The software can be deployed in a folder like C:appTailFlickTest. There are two subfolders:
C:appTailFlickTestbin contains executables
C:appTailFlickTestdoc contains documentations
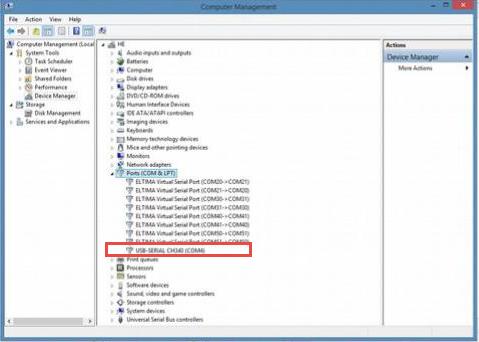
UBS to 232 driver install on the software machine is required. The driver is included in the cable box.

When the tail flick hardware is connected to the computer via the USB cable, a port is created as shown on the Device Manager:
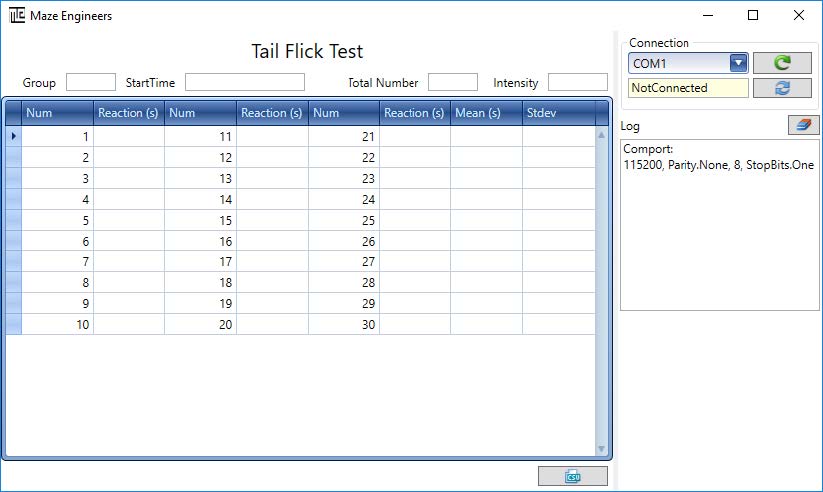
Step 1: Double click on the main application executable
C:appTailFlickTestbinTailFlickUI.exe
The application window appears as below:
 Step 2: Once the computer recognizes the hardware, choose the comport that matches the one on the Device Manager, and then click on button.
Step 2: Once the computer recognizes the hardware, choose the comport that matches the one on the Device Manager, and then click on button.
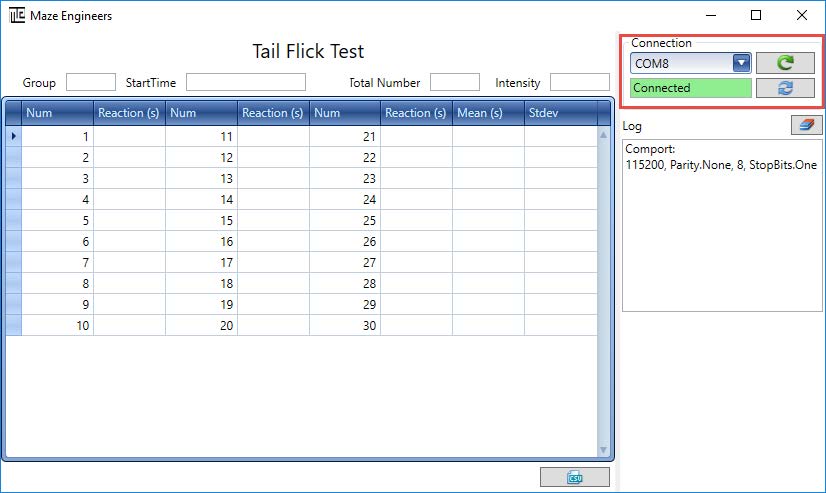
Note that the comport Reload button allows user to reload the comport list if the device is connected to computer after the software has been started.
Step 3: On the device screen, browse to the group data to upload and then press Print button on the screen
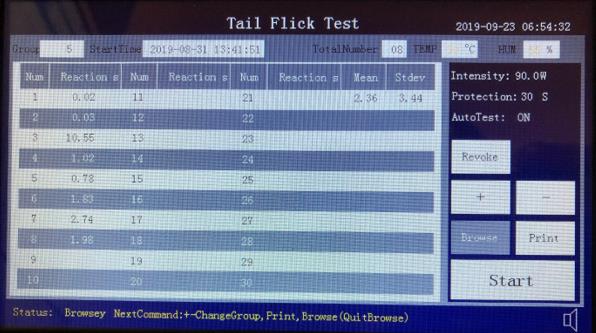 The data will be uploaded to the software screen:
The data will be uploaded to the software screen:
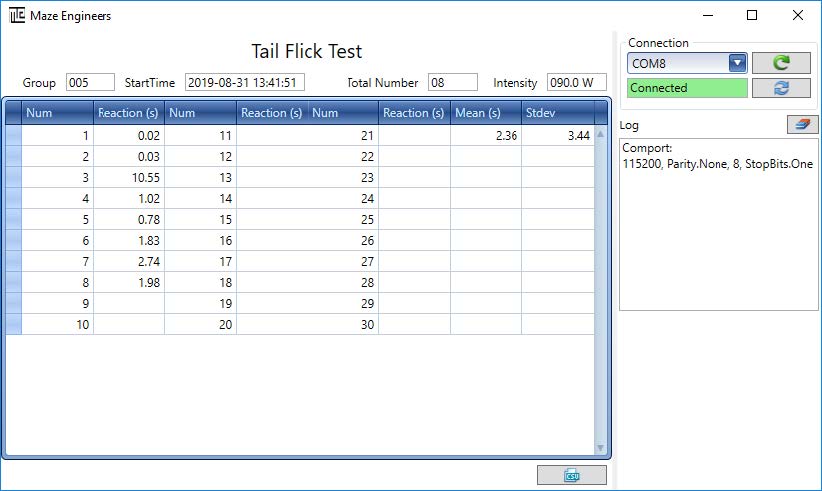
Step 4: Export data to CSV file
Click button on the software screen, a file chooser appears for user to save the data with CSV data format. The default folder is C:appTailFlickTestbinuser_data.
The screen is capacitive touch screen, able to achieve 99% accuracy, with a response speed of less than 3ms, smooth user experience, to meet user’s operating habits of touch screen products
The screen has the advantages of clear display, true color and high contrast. The interface design has a strong sense of hierarchy, reasonable layout and simple operation.

A laser sensor is used as a reaction detection of the animal’s tail moving away from the light source, and the detection time is less than 0.01S.
The light power can be set to a range of 20w-100w with a step of adjustment 0.5w and with precise control of the illumination power through pulse width modulation.
A high-grade filter lens is used to partially absorb visible light while maximizing heat radiation. This is to achieve good experimental results and reduce the visual interference of glare to the experimenter.
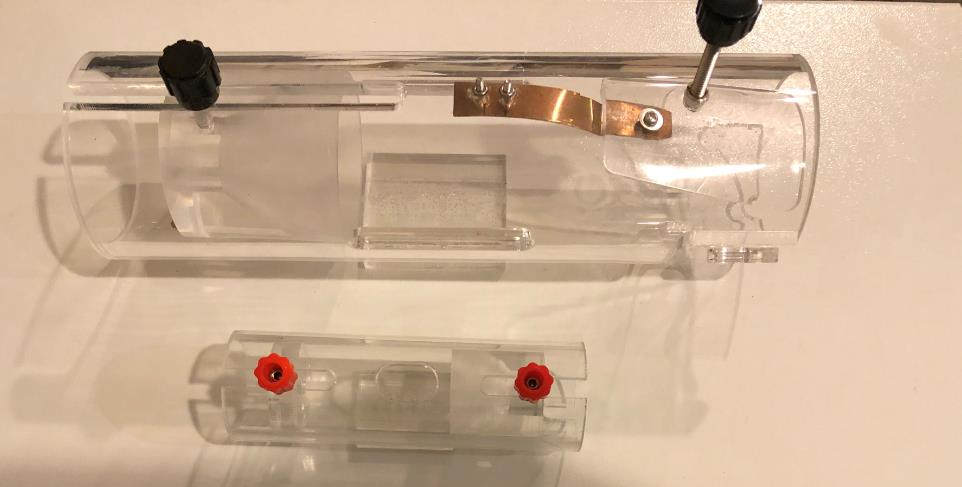
A mouse refrainer and a rat refrainer are provided. A video is included to show how to use the animal refrainer.

Turn on power, the main screen appears as shown below:
Delete data Press button will delete all experiment data and experiment group is reset to 1.
Start: Press button will enter experiment screen
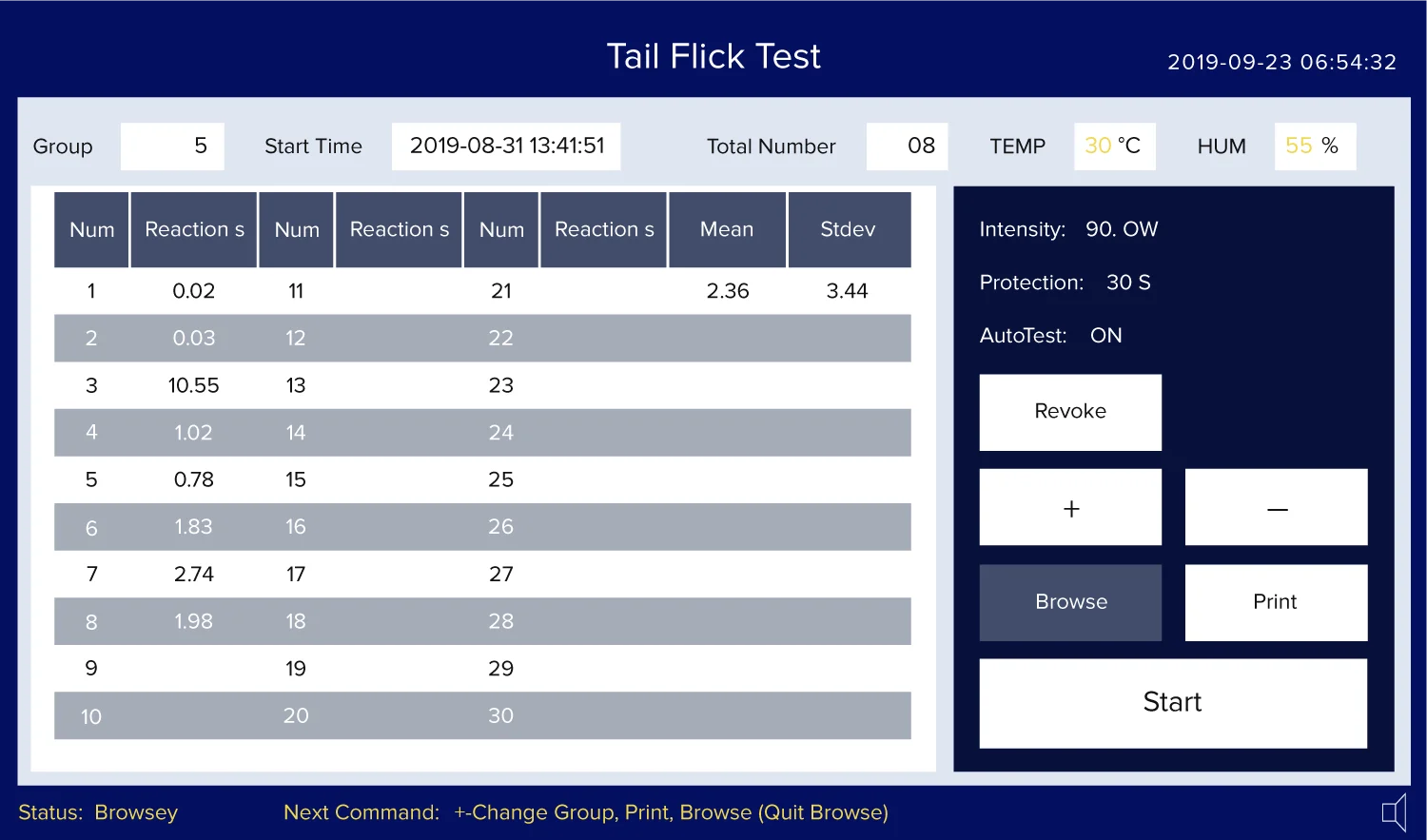
When the experiment screen first appears, it shows last experiment data by default.
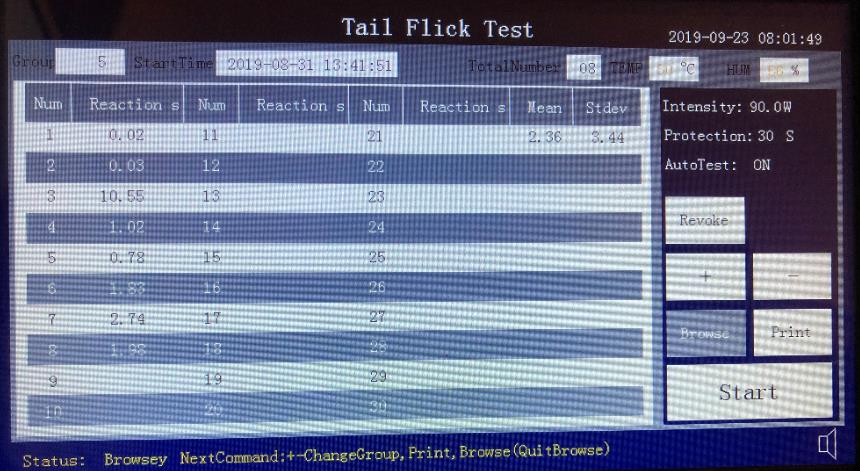
Press start button on the experiment screen to start the experiment
Put animal tail on top of the light and let the tail on the laser sensor
Press foot switch to open the heat light. This automatically starts time counting
When the animal feels pain and flick its tail,
Repeat the experiment according to your protocol
Press Stop to finish the experiment (of this group)
If AutoTest is on (in the auto mode), the time counting stops by the laser sensor
If AutoTest is off (in the manual mode), the time counting stops by pressing foot switch
Either case, foot switch is required to start the heat light
After the experiment, press Browse to navigate the experiment data, Use + and – to go to next or previous group. The device can save up to 200 groups of data.
After the experiment, press Print button to print the group data to the printer.
If the apparatus is connected to a computer, the data can be uploaded to PC. See Tail Flick Data Upload Software Manual.pdf for more detail.
This software provides an UI application for user to upload Tail Flick test data to PC.
The software can be deployed in a folder like C:appTailFlickTest. There are two subfolders:
C:appTailFlickTestbin contains executables
C:appTailFlickTestdoc contains documentations
UBS to 232 driver install on the software machine is required. The driver is included in the cable box.

When the tail flick hardware is connected to the computer via the USB cable, a port is created as shown on the Device Manager:

Step 1: Double click on the main application executable
C:appTailFlickTestbinTailFlickUI.exe
The application window appears as below:
 Step 2: Once the computer recognizes the hardware, choose the comport that matches the one on the Device Manager, and then click on button.
Step 2: Once the computer recognizes the hardware, choose the comport that matches the one on the Device Manager, and then click on button.

Note that the comport Reload button allows user to reload the comport list if the device is connected to computer after the software has been started.
Step 3: On the device screen, browse to the group data to upload and then press Print button on the screen
 The data will be uploaded to the software screen:
The data will be uploaded to the software screen:

Step 4: Export data to CSV file
Click button on the software screen, a file chooser appears for user to save the data with CSV data format. The default folder is C:appTailFlickTestbinuser_data.
The screen is capacitive touch screen, able to achieve 99% accuracy, with a response speed of less than 3ms, smooth user experience, to meet user’s operating habits of touch screen products
The screen has the advantages of clear display, true color and high contrast. The interface design has a strong sense of hierarchy, reasonable layout and simple operation.

A laser sensor is used as a reaction detection of the animal’s tail moving away from the light source, and the detection time is less than 0.01S.
The light power can be set to a range of 20w-100w with a step of adjustment 0.5w and with precise control of the illumination power through pulse width modulation.
A high-grade filter lens is used to partially absorb visible light while maximizing heat radiation. This is to achieve good experimental results and reduce the visual interference of glare to the experimenter.
A mouse refrainer and a rat refrainer are provided. A video is included to show how to use the animal refrainer.



Turn on power, the main screen appears as shown below:

Delete data Press button will delete all experiment data and experiment group is reset to 1.
Start: Press button will enter experiment screen
When the experiment screen first appears, it shows last experiment data by default.

Press start button on the experiment screen to start the experiment
Put animal tail on top of the light and let the tail on the laser sensor
Press foot switch to open the heat light. This automatically starts time counting
When the animal feels pain and flick its tail,
Repeat the experiment according to your protocol
Press Stop to finish the experiment (of this group)
If AutoTest is on (in the auto mode), the time counting stops by the laser sensor
If AutoTest is off (in the manual mode), the time counting stops by pressing foot switch
Either case, foot switch is required to start the heat light
After the experiment, press Browse to navigate the experiment data, Use + and – to go to next or previous group. The device can save up to 200 groups of data.
After the experiment, press Print button to print the group data to the printer.
If the apparatus is connected to a computer, the data can be uploaded to PC. See Tail Flick Data Upload Software Manual.pdf for more detail.
Carefully position the subject into the restraining chamber and give it ample time to adjust to the environment. Repeat this process as needed to ensure the subject is relaxed and stable before conducting the actual trial.
Position the subject in the restraining compartment and let it acclimate for 15 to 20 minutes. To enhance heat absorption, you may paint the test area of the tail black. Place the tail directly under the heat source, then start both the timer and the heat source simultaneously. Monitor the subject for signs of pain until the pre-set cut-off time, typically between 20 and 35 seconds, is reached. Conduct additional trials as needed, ensuring a minimum 5-minute break between each trial.
Haddadi et al. investigated the impact of various doses of carvacrol on pain responses in adult male Wistar rats. The study involved 6-OHDA-lesioned rats divided into four groups receiving carvacrol at doses of 25 mg/kg, 50 mg/kg, and 100 mg/kg, with an additional control group and a sham-operated group. Each rat underwent three trials of 15 seconds, where different sections of the tail were exposed to a heat stimulus. Compared to the sham group, the lesioned rats exhibited a notable reduction in tail flick latency. However, carvacrol treatment did not produce a significant change in tail flick latency.
Li et al. categorized rats into nine groups, each receiving an intraperitoneal injection of either 0.9% normal saline, “n” (at doses of 2.5 mg/kg and 5 mg/kg), “a” (at doses of 42, 84, and 168 mg/kg), or various combinations of “n” and “a” (e.g., 1.25 N + 21 A, 2.5 N + 42 A, 5.0 N + 84 A mg/kg; n=9, 9, and 8 respectively) before conducting the tail flick test. Tail flick latencies were measured at five 15-minute intervals during a 30-second trial. The findings demonstrated a dose-dependent effect of the drugs on tail flick latencies. Notably, the combination of the two drugs produced a more pronounced antinociceptive effect compared to the effects of nefopam alone at equivalent doses.
Nealon et al. used experimentally naïve adult male and female C57BL/6N mice and divided them into two diet groups. One group was maintained on a high-fat, energy-dense, while the other group was maintained on standard chow for 15-weeks before the testing and drug administration. The subjects were treated with 0 (saline), 1, 3, 10, and 30 mg/kg “m”and tested for 10 seconds in the tail flick test. A dose-dependent increase in tail withdrawal latencies was observed. A difference in the magnitude of responses was observed between the two sexes, with females on either diet showing less sensitivity to effects of “m”.
In the Tail Flick test, the time taken for a tail to withdraw in response to a radiant heat stimulus is recorded. Withdrawal latencies can be assessed for various segments of the tail. It is crucial to distinguish reflexive behavior from voluntary actions during the observation. If any ambiguous behavior is noted, the experiment should be repeated to accurately determine and document the withdrawal latency.
Carefully position the subject into the restraining chamber and give it ample time to adjust to the environment. Repeat this process as needed to ensure the subject is relaxed and stable before conducting the actual trial.
Position the subject in the restraining compartment and let it acclimate for 15 to 20 minutes. To enhance heat absorption, you may paint the test area of the tail black. Place the tail directly under the heat source, then start both the timer and the heat source simultaneously. Monitor the subject for signs of pain until the pre-set cut-off time, typically between 20 and 35 seconds, is reached. Conduct additional trials as needed, ensuring a minimum 5-minute break between each trial.
Haddadi et al. investigated the impact of various doses of carvacrol on pain responses in adult male Wistar rats. The study involved 6-OHDA-lesioned rats divided into four groups receiving carvacrol at doses of 25 mg/kg, 50 mg/kg, and 100 mg/kg, with an additional control group and a sham-operated group. Each rat underwent three trials of 15 seconds, where different sections of the tail were exposed to a heat stimulus. Compared to the sham group, the lesioned rats exhibited a notable reduction in tail flick latency. However, carvacrol treatment did not produce a significant change in tail flick latency.
Li et al. categorized rats into nine groups, each receiving an intraperitoneal injection of either 0.9% normal saline, "n" (at doses of 2.5 mg/kg and 5 mg/kg), "a" (at doses of 42, 84, and 168 mg/kg), or various combinations of "n" and "a" (e.g., 1.25 N + 21 A, 2.5 N + 42 A, 5.0 N + 84 A mg/kg; n=9, 9, and 8 respectively) before conducting the tail flick test. Tail flick latencies were measured at five 15-minute intervals during a 30-second trial. The findings demonstrated a dose-dependent effect of the drugs on tail flick latencies. Notably, the combination of the two drugs produced a more pronounced antinociceptive effect compared to the effects of nefopam alone at equivalent doses.
Nealon et al. used experimentally naïve adult male and female C57BL/6N mice and divided them into two diet groups. One group was maintained on a high-fat, energy-dense, while the other group was maintained on standard chow for 15-weeks before the testing and drug administration. The subjects were treated with 0 (saline), 1, 3, 10, and 30 mg/kg “m”and tested for 10 seconds in the tail flick test. A dose-dependent increase in tail withdrawal latencies was observed. A difference in the magnitude of responses was observed between the two sexes, with females on either diet showing less sensitivity to effects of “m”.
In the Tail Flick test, the time taken for a tail to withdraw in response to a radiant heat stimulus is recorded. Withdrawal latencies can be assessed for various segments of the tail. It is crucial to distinguish reflexive behavior from voluntary actions during the observation. If any ambiguous behavior is noted, the experiment should be repeated to accurately determine and document the withdrawal latency.
The Tail Flick test assesses the subject’s response to thermal nociception. Unlike other tests, such as the Hot Plate test and the Hargreaves test, the Tail Flick test does not expose the animal to a heated open field or concentrate the stimulus on the plantar surface. Instead, it measures withdrawal latencies by applying heat to different areas of the tail. This test is straightforward and easy to conduct, offering two variations to accommodate different research budgets.
In spinally transacted subjects, the tail flick response may be a spinal reflex rather than pain response (Irwin et al., 1951). Thus, consideration of the subject’s motor function (Chapman et al., 1985) is vital in achieving accurate results. The observer should be able to differentiate voluntary movement from pain reflex behaviors. Since Tail Flick test requires restraining, it is essential that the subjects are well accustomed to that process. Restraining may also cause anxiety-related behaviors. The cut-off time should not exceed 30 to 35 seconds to avoid unnecessary harm to the subject. It is important to minimize any external stimulus in the test arena to reduce their impact on the subject’s performance. Handling and consistency of the surgical process can also potentially influence tail withdrawal responses. The tail flick test may not be successful in distinguishing between opioid agonists and mixed agonist-antagonists. However, this can be overcome by using a cold-water Tail Flick test (Pizziketti et al., 1985).
The Tail Flick test assesses the subject's response to thermal nociception. Unlike other tests, such as the Hot Plate test and the Hargreaves test, the Tail Flick test does not expose the animal to a heated open field or concentrate the stimulus on the plantar surface. Instead, it measures withdrawal latencies by applying heat to different areas of the tail. This test is straightforward and easy to conduct, offering two variations to accommodate different research budgets.
In spinally transacted subjects, the tail flick response may be a spinal reflex rather than pain response (Irwin et al., 1951). Thus, consideration of the subject’s motor function (Chapman et al., 1985) is vital in achieving accurate results. The observer should be able to differentiate voluntary movement from pain reflex behaviors. Since Tail Flick test requires restraining, it is essential that the subjects are well accustomed to that process. Restraining may also cause anxiety-related behaviors. The cut-off time should not exceed 30 to 35 seconds to avoid unnecessary harm to the subject. It is important to minimize any external stimulus in the test arena to reduce their impact on the subject’s performance. Handling and consistency of the surgical process can also potentially influence tail withdrawal responses. The tail flick test may not be successful in distinguishing between opioid agonists and mixed agonist-antagonists. However, this can be overcome by using a cold-water Tail Flick test (Pizziketti et al., 1985).
Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE (1985). Pain measurement: an overview. Pain. 22(1):1-31.
D’Amour F. E., Smith D. L. (1941). A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 72, 74–79.
Deuis JR, Dvorakova LS, Vetter I (2017). Methods Used to Evaluate Pain Behaviors in Rodents. Front Mol Neurosci. 10:284. doi: 10.3389/fnmol.2017.00284
Haddadi H, Rajaei Z, Alaei H, Shahidani S (2018). Chronic treatment with carvacrol improves passive avoidance memory in a rat model of Parkinson’s disease. Arq Neuropsiquiatr. 76(2):71-77. doi: 10.1590/0004-282X20170193.
Irwin S, Houde R.W, Bennett D.R, Hendershot L.C, Seevers M.H (1951). The effects of “m”e and “m”on some reflex responses of spinal animals to nociceptive stimulation. J Pharmacol Exp Ther. 101(2):132-43.
Li Q, Zhuang Q, Gu Y, Dai C, Gao X, Wang X, Wen H, Li X, Zhang Y (2018). Enhanced analgesic effects of “n” in combination with “a” in rodents. Biomed Rep. 2018 Feb;8(2):176-183. doi: 10.3892/br.2017.1032.
Nealon CM, Patel C, Worley BL, Henderson-Redmond AN, Morgan DJ, Czyzyk TA (2018). Alterations in nociception and “m” antinociception in mice fed a high-fat diet. Brain Res Bull. 138:64-72. doi: 10.1016/j.brainresbull.2017.06.019.
Pizziketti RJ, Pressman NS, Geller EB, Cowan A, Adler MW (December 1985). “Rat cold water tail-flick: a novel analgesic test that distinguishes “o” agonists from mixed agonist-antagonists“. Eur. J. Pharmacol. 119 (1–2): 23–9. doi:10.1016/0014-2999(85)90317-6.
No questions yet for this product.
