$990.00 – $1,490.00Price range: $990.00 through $1,490.00
The Maze Engineers Head fixation system is utilized in neurobiological research settings to stabilize mouse head movements across various behavioral experiments. The body cover and lid system allow for easy transfer of the mouse to the system. Customizable head bars allow for versatility for stabilization and application.
These devices are frequently employed in the study of anaesthesia effects, facial function, neuroimaging, reflex adaptation, operant conditioning, and behaviors such as eye blinking in mice (Schwarz et al., 2010).

MazeEngineers empowers preclinical neuroscience research with meticulously designed, customizable behavioral apparatuses. From manual classic mazes to fully automated smart systems, we provide the tools scientists need to capture high-quality, reproducible data for studies on learning, memory, anxiety, and depression.

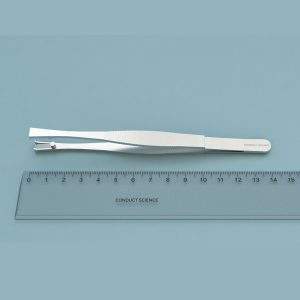

Mouse Head fixation system Features | |
Body cover and lid: 102mm L x 34 mm W x 38.5 mm H | |
Head plate default (customizable upon request): Skull plate window 6 mm W x 6 mm L, Overall default dimensions: 15.8 mm L x 24mm W |
Head fixation serves as a fundamental procedure in neurological research. Its primary role is to immobilize subjects, thereby facilitating optical assessment of neuronal circuits and reducing background noise and motion artifacts. The head fixation system limits the range of behavioral movements, crucial for studying learning behaviors related to reward and specific motor skills such as eye movements, lever pulling, licking, and engaging in virtual reality scenarios (Weaver et al., 2023).
Moreover, head fixation devices are extensively utilized in neuroscience laboratories for recording neuronal activity through techniques such as calcium imaging and electrophysiology.
The process of head fixation is essential as it influences head and eye movements in response to visual stimuli. Beyond motor reflex studies, head fixation devices are instrumental in training mice for operant conditioning tasks and facilitating differentiation between auditory, visual, and olfactory stimuli.
To implement head fixation, the head bar must be securely attached to the animal’s head, followed by immobilization of the head bar and the subject’s head using an external apparatus. A head hat is also used to protect hardware mounted on the skull.
The head fixation system features a metal restrainer platform and removeable lid, allowing for easy movement of the subject. This restrainer ensures superior stability and comfort for the subject throughout the experiment. Thumbscrews allow easy capture and release of the animal.
The default aluminum headplate has a 6 mm x 6 mm skull window and is fixed with M2.5 cross countersunk screws.
Customizations include material composition and dimensions, please enquire
Mazeengineers provide an ergonomically designed head fixation system tailored to research needs.
The following can be studied using the Rodent Head-Fixation apparatus:
Hughes et al. (2020) tried studying the subtle movement and postural adjustments that animals experience during head fixation. The experimenters utilized a novel mouse head fixation system equipped with five orthogonal force sensors to study in vivo electrophysiology of the mouse brain during behavioral tasks like licking.
They housed the mice in groups of 3-4 mice per cage following a 12:12 light cycle and conducted the experiments in the light phase. The subjects were deprived of water and allowed free access to water for about 2h after the experimental sessions. The scientists attached the mouse perch and head fixation apparatus to a base plate made of steel and elevated this assembly to accommodate the reward delivery apparatus. The load cells were also added to the experimental setup. Following this, the researchers anesthetized the mice with 2-3% isoflurane and performed craniotomy using a stereotaxic frame. During the surgery, they implanted 16 electrodes in a 4×4 configuration using thumbscrews and secured them using dental acrylic. Then, they also inserted a metal bar and cemented it using dental acrylic.
Ultimately, the experimenters trained the mice for a Pavlovian conditioning task and observed the licking behavior. They concluded that licking-related oscillations revealed postural alterations among the subjects.
The head fixation system is a highly effective tool featuring an ergonomic design utilized in neurobiology laboratories to secure murine heads. A primary advantage of these devices is their ability to facilitate precise monitoring of neuronal activity in mice, thereby minimizing background noise and motion artifacts. The mechanical stability provided by head fixation creates optimal conditions for visualizing neural activity in awake animals. Additionally, these devices offer researchers greater experimental control and ensure electrophysiological neural signals remain unaffected even without task-specific training (Schwarz et al., 2010). Modern head fixation systems also occupy minimal space on the subject’s head.
However, there are several drawbacks to consider. For example, acclimating a head-fixed behaving rat to the experimental setup can be time-consuming. Furthermore, the system restricts the animal’s ability to perform extensive whole-body movements. Lastly, head bars wider than the subject’s body may require extended time for effective head fixation.
A head fixation system is employed in neurobiology laboratories to affix mouse heads during several behavioral paradigms.
Guo, Z. V., Hires, S. A., Li, N., O’Connor, D. H., Komiyama, T., Ophir, E., … & Svoboda, K. (2014). Procedures for Behavioral Experiments in Head-Fixed Mice. PLoS ONE, 9(2).
Hughes, R. N., Bakhurin, K. I., Barter, J. W., Zhang, J., & Yin, H. H. (2020). A head-fixation system for continuous monitoring of force generated during behavior. Frontiers in integrative neuroscience, 14, 11.
Schwarz, C., Hentschke, H., Butovas, S., Haiss, F., Stüttgen, M. C., Gerdjikov, T. V., … & Waiblinger, C. (2010). The head-fixed behaving rat—procedures and pitfalls. Somatosensory & motor research, 27(4), 131-148.
Weaver, I. A., Yousefzadeh, S. A., & Tadross, M. R. (2023). An open-source head-fixation and implant-protection system for mice. HardwareX, 13, e00391.
| Dimensions | N/A |
|---|---|
| Species | Mouse, Rat |