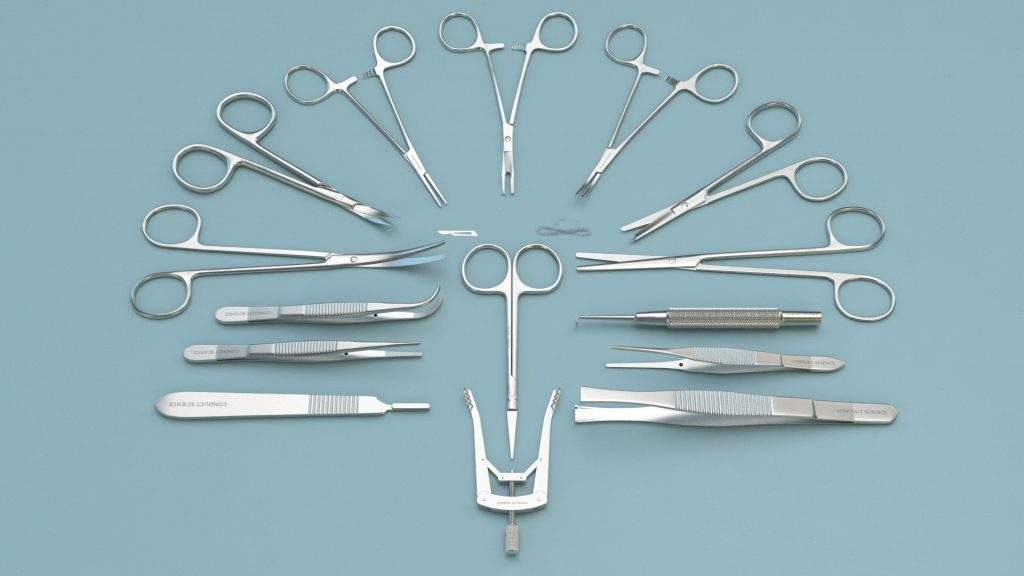
Mouse Kit
| SKU |
Product Description |
Qty |
| S12003-09 |
IRIS-Fine Scissors (Round Type)-S/S Str/24.5*5mm/9cm |
1 |
| S12004-09 |
IRIS-Fine Scissors (Round Type)-S/S Cvd/24*4.6mm/9.5cm |
1 |
| S13003-11 |
STEVENS Fine Dissecting Scissors (Flat Type)-B/B Str/25*4mm/11.5cm# |
1 |
| S13004-11 |
STEVENS Fine Dissecting Scissors (Flat Type)-B/B Cvd/28*5mm/11.5cm |
1 |
| S15001-09 |
SPENCER Ligature Scissors (Slender Type)-20.3*4mm/9cm# |
1 |
| S32003-12 |
Scalpel Handles with Ruler 3# Solid-12.5cm |
1 |
| S31015-01 |
15# Scalpel Blades (Box of 100pcs) |
1 |
| F12005-10 |
IRIS Dissecting Forceps-Str, Tip width 1mm, teeth length 14mm, 10cm |
1 |
| F12006-10 |
IRIS Dissecting Forceps-Light Cvd, Tip width 1mm, teeth length 13.5mm, 10cm |
1 |
| F22002-10 |
HARTMAN Mosquito Forceps-Str/20*0.8mm/10.5cm |
1 |
| F22003-10 |
HARTMAN Mosquito Forceps-Cvd/20.8*1mm/10cm |
1 |
| F13029-10 |
IRIS 1x2 Teeth Tissue Forceps-Str, Tip width 1.3mm, 10.5cm |
1 |
| F31047-12 |
OLSEN-HEGAR Needle Holders with Scissors-Str, 10*2.15mm/12cm |
1 |
| F35305-50 |
Absorbable PGA Sutures w/Needle-○1/2/4×10/90㎝/5-0 (50/Box |
1 |
| R22005-45 |
3x3 Teeth Retractors-Blunt, 30mm Spread,5cm |
1 |
| R31005-04 |
SS Micro Clamps-Str/L*W 4*0.75mm/16mm |
1 |
| R42002-12 |
Spinal Cord Hook - Tip Dia. 3mm/12cm# |
1 |
| R34001-14 |
Clip Applicator for R31005- and R31006-Clamps-8.4*5mm/14cm |
1 |
| SP0000-P |
Instrument Storage Portfolio, 32*22cm |
1 |
Rat Kit
| SKU |
Product Description |
Qty |
| S12005-10 |
IRIS-Fine Scissors (Round Type)-S/S Str/10.5cm |
1 |
| S12006-10 |
IRIS-Fine Scissors (Round Type)-S/S Cvd/10.5cm |
1 |
| S12010-11 |
IRIS-Fine Scissors (Round Type)-S/S Cvd/11.5cm |
1 |
| S13005-14 |
Dissecting Scissors (Round Type)-B/B Str/14.5cm |
1 |
| S13006-14 |
Dissecting Scissors (Round Type)-B/B Cvd/14.5cm |
1 |
| F21001-12 |
HALSTED Artery Forceps-Str, 2.0mm Tips, 12.5cm |
2 |
| F21002-12 |
HALSTED Artery Forceps-Cvd, 2.0mm Tips, 12.5cm |
2 |
| F12010-10 |
Dressing Forceps-Str, 1.9mm Tips, 10.5cm |
2 |
| F12011-10 |
Dressing Forceps-Cvd, 1.9mm Tips, 10.5cm |
2 |
| F13029-10 |
IRIS 1x2 Teeth Tissue Forceps-Str, 0.8mm Tips, 10cm |
1 |
| F31047-12 |
OLSEN-HEGAR Needle Holders with Scissors-Str, 12cm |
1 |
| S15001-11 |
SPENCER Ligature Scissors (Slender Type-11cm |
1 |
| S32001-12 |
Scalpel Handles 3# Solid-12cm |
1 |
| S31015-01 |
15# Scalpel Blades (Box of 100pcs) |
1 |
| F35305-50 |
PGA Sutures w/Needle-○1/2/4×10/90㎝/5-0 (50/Box |
10 |
| R22009-01 |
ALM 4x4 Teeth Retractors-Blunt, 7cm |
1 |
| R31005-06 |
SS Micro Clamps-Str/L*W 6*1mm/15mm |
5 |
| R42002-12 |
Spinal Cord Hook - Tip Dia. 3mm/12cm |
1 |
| R34001-14 |
Applicator for R31005- and R31006-Clamps-14cm |
1 |
| SP0000-P |
Instrument Storage Portfolio, 32*22cm |
1 |
Small Animal Kit
| SKU |
Product Description |
Qty |
| S12009-11 |
RIS-Fine Scissors (Round Type)-S/S Str/11.5cm |
1 |
| S12010-11 |
IRIS-Fine Scissors (Round Type)-S/S Cvd/11.5cm |
1 |
| S12031-11 |
Standard Scissors (Round Type)-S/S Full Cvd/11.5cm |
1 |
| S13005-14 |
Dissecting Scissors (Round Type)-B/B Str/14.5cm |
1 |
| S13006-14 |
Dissecting Scissors (Round Type)-B/B Cvd/14.5cm |
1 |
| F12010-13 |
Dressing Forceps-Str, 2.1mm Tips, 13cm |
2 |
| F12011-13 |
Dressing Forceps-Cvd, 2.1mm Tips, 13cm |
2 |
| F21001-12 |
HALSTED Artery Forceps-Str, 2.0mm Tips, 12.5cm |
2 |
| F21002-12 |
HALSTED Artery Forceps-Cvd, 2.0mm Tips, 12.5cm |
2 |
| S15001-11 |
SPENCER Ligature Scissors (Slender Type-11cm |
1 |
| S32001-12 |
Scalpel Handles 3# Solid-12cm |
1 |
| S31015-01 |
15# Scalpel Blades (Box of 100pcs) |
1 |
| F13014-12 |
1x2 Teeth Tissue Forceps-Str, 1.0mm Tips, 12.5cm |
1 |
| F31047-14 |
OLSEN-HEGAR Needle Holders with Scissors-Str, 14cm |
1 |
| F35305-50 |
PGA Sutures w/Needle-○1/2/4×10/90㎝/5-0 (50/Box |
10 |
| R22015-02 |
WULLSTEIN 3x3 Teeth Retractors-Blunt, 13cm |
1 |
| R31008-26 |
Schwartz Micro Clamps-Str/26mm |
5 |
| R42002-12 |
Spinal Cord Hook - Tip Dia. 3mm/12cm |
1 |
| SP0000-P |
Instrument Storage Portfolio, 32*22cm |
1 |
Introduction
Dissection is a Latin word meaning “to cut to pieces.” It is the process of disassembling the body parts of laboratory animals for research purposes. The dissection is an exciting and illuminating aspect of medical science, allowing the researchers to learn the complex anatomical structures of the laboratory animals as well as enabling them to perform experimental manipulations for biomedical research.
Basic biomedical research includes the characterization of genes/proteins, the study of anatomical structures and physiological functions, and the identification of normal and pathological states in a variety of animal species. This knowledge is then employed to understand these same processes in humans. Likewise, the information obtained in the field of human medicine can be mined to advance veterinary medicine because of the commonalities among species that form the basis of comparative medicine. Rodents are the most preferred species to be used as animal models for biomedical research due to their anatomical, physiological, and genetic similarities to humans. Unlike larger animals, the advantages of rodents include their small size, ease of maintenance, shorter life cycle, and abundant genetic resources.
Pre-operative Set-up and Euthanasia
Rodents are euthanized before the dissection procedures. Different methods of euthanasia are applied, enabling a rapid death with reduced pain for the animal as well as the safety of the field workers. Recommended physical methods for euthanizing the rodents include:
Cervical Dislocation
Cervical dislocation is one of the widely used procedures for euthanizing. The protocol aims at dislocating the cervical vertebrae from the skull quickly. The technique ensures a rapid loss of consciousness and makes sure that dislocation is cervical and not lower in the vertebral column. The cervical dislocation is recommended to be used only for small-sized rodents (mice and small rats). This method should be applied if there are a few animals to be euthanized to prevent human error due to fatigue. It includes pulling the head from the body to dislocate the neck with a sudden jerk. The method is described as follows:
- Hold the head with one hand and place the thumb and the index finger on either side of the neck at the base of the skull.
- Pull the base of the tail with the other hand quickly.
- Just press the thumb tip firmly into the neck behind the skull immediately.
Cervical dislocation is usually applied to animals that can be handled easily. However, as wild or large animals become aggressive and more stressed by human contact, the following methods are recommended to euthanize the larger animals.
Carbon Dioxide Inhalation
- Place the cage containing the animal in a chamber with a flow of 100% CO2 gas initiated.
- Monitor the flow of CO2 gas using a gas flow meter to reach 20-30% of chamber volume per minute (a higher flow rate may result in animal stress due to pain before the loss of consciousness, whereas a lower rate would be too slow).
The euthanasia method employing CO2 is inexpensive, non-flammable, non-explosive, and poses a minimal hazard to the handler when assisted with properly designed equipment.
Overdose of Inhalant Anesthetic Agents
While euthanizing the animals using inhalant anesthetic agents, euthanize in open-air places, far from the other captured animals. Enflurane, isoflurane, sevoflurane, methoxyflurane, and desflurane with or without nitrous oxide are recommended. As these anesthetic agents are non-flammable and non-explosive under ordinary environmental conditions. Chloroform and ether are not satisfactory because chloroform is toxic and recognized as carcinogenic and ether is irritating, flammable, and requires an extended waiting time before processing the animal. Use an appropriate hermetic container resisting the agents to store the anesthetic agents.
Dissection Protocols
- Place the animal with the ventral side up on a clean dissection board and pin it. Avoid holding the animal in one hand to keep your hands clean and to cut the organs accurately.
- Using soaked cotton, clean the ventrum to avoid the introduction of animal hair into the body that may infect the organs.
- Hold the skin with dissecting forceps and raise it above the abdomen.
- Make an incision through the body wall muscles by putting the scissor just anterior to the genital opening and continue cutting on one side of the midline, ventral to the thoracic cavity.
Note: blunt-ended scissors (either two blunt-end scissors or one blunt, one sharp point scissors) are recommended to prevent organ damage.
- Keep the fur away from the body by pulling the skin from both sides of the thoracic cavity.
- Isolate the larger parts of the lungs and store them in the cryogenic tubes quickly.
- Clean the forceps and scissors using a solution containing bleach, water, and ethanol.
- Find the spleen by moving the stomach to the left (the spleen has a triangular shape).
- Pull the spleen out by holding it with the forceps and get rid of the white tissue with the help of sharp pointy scissors.
- Clean the forceps and scissors with the cleaning solution.
- Displace the bowels to find the kidneys beneath the intestines.
- Separate the kidneys and place them in two cryogenic tubes for storage (if the kidneys are too big, cut them into pieces).
- Clean the dissecting instruments with the cleaning solution.
- Dissect the diaphragm and keep it in a cryopreservative tube.
- Place the cryogenic tubes containing the dissected organs in a liquid nitrogen tank (Do not open the tank again and again rather put the tubes at one time to prevent nitrogen evaporation from the tank).
- Wash the forceps and scissors with the solution containing bleach, water, and ethanol).
- Identify the sex of the animal by observing the genital organs and counting the embryos or measuring the size of the testes.
- Tag the leg of the animal. Using fine point forceps, perforate the skin and introduce the string.
- Place the animal into an ethanol-filled jar.
- Clean and wash the dissection board and the operative area.
Dissection of Mouse EDL and Soleus Muscles (Bröllochs, 2017)
- Fix the leg of the animal with pins in a flexed position to the dissecting dish and cover the leg with Ringer solution.
- Remove the skin of the leg and get rid of the excessive fascia surrounding the muscles.
- With the help of the forceps, hold the Achilles tendon. Use the bodkin side of the scissors to rub the gap open between the Achilles tendon and the other tendons. Cut the Achilles tendon as close as possible to the foot when the gap is visible.
- As the gap starts to open, support that gap to open up fully.
- The Soleus muscle will be visible as the gap opens up to the knee. The Soleus muscle is dark pink, identify it by its color. Carefully cut the tendon near the knee.
- Hold the Soleus muscle from its upper tendon gently with the forceps and pull it down slowly and carefully while (if needed) removing the surrounding fascia that is still holding it. Cut the lower tendon after freeing the whole muscle from the fascia.
- Isolate the Soleus muscle from the leg and remove the leftover fascia. For more comfortable handling, one should move the muscle to a new dissecting dish, fix it with pins, infuse it with Ringer solution, and store it at 4 °C.
- To dissect the EDL muscle, place and pin the leg into a stretched position first and remove the fascia.
- Rub the lower leg’s tendons. The 4 EDL tendon ends are next to the V-looking Tibialis tendon. Carefully lift the tendons to observe the EDLs. The toes of the paw stretch while lifting. Make an incision at the ends of the tendon as close as possible to the foot.
- A pocket will open up as the ends are cut. Remove the surrounding fascia. Isolate the EDL muscles from the pocket to visualize the upper tendon. Dissect the upper tendon then.
- Isolate the EDL muscle from the leg and remove all the fascia and the fat.
- Place both the dissected muscles in a clean dissecting dish. Lightly stretch the muscles and pin them. Cover the dissected muscles with a 3.7% PFA.
- Store the dish at 4°C overnight after covering it with the parafilm.
Gross Postmortem Location and Examination of Heart, Lungs, Liver, Kidneys, and Spleen Method (Parkinson et al., 2011)
- Examine the animal for skin and coat abnormalities, emaciation, or dehydration.
- Keep a log of artificial manipulations, implants, or surgical scarring, if any.
- With the help of the dissecting microscope examine all the external orifices (ears, eyes, nose, anus, genital openings, and oral cavity) for secretion or bleeding.
- Place the euthanized animal in dorsal recumbency on a clean dissection board.
- With the help of dissection scissors, make an incision through the skin from the length of the ventrum extending from the anus to the chin, reflecting the skin and incising the abdominal wall, opening the abdominal viscera, salivary and preputial/clitoral glands, and cervical and axillary lymph nodes. Examine the thoracic viscera by making 2 incisions laterally up on each side of the ribcage, then make a cut across, at the top of the sternum, to open a wide space enough to observe all the lobes of the lung thoroughly.
- Observe the musculoskeletal structure.
- Assess the condition of all the organs for abnormalities. Locate and identify the heart and the lungs in the thoracic cavity. Observe the liver, kidneys, and spleen in the abdominal cavity. Check the organs for any color changes, size differences, and absence or dislocation. Record the consistency of surfaces, any additional tissue (e.g., masses), fluid pockets, or the presence of fluid in the abdominal/thoracic cavities.
- Examine the gastrointestinal tract for contents, lack of contents, thickened walls, masses, blood clots, and hemorrhage. Using a sharp blade, cut the kidneys (left-longitudinal section, right-cross section, on the midline, but off-center) to assess parenchyma for any abnormality. Check the presence of any large lymph nodes on the mesentery.
- Observe the urogenital system and check for any blockage, fluid pockets, hemorrhage, or other abnormalities.
Postmortem Collection of Heart, Liver, Kidneys, and Spleen for Histopathology
- Collect a container filled with a suitable amount of 10% neutral buffered formalin (NBF), and make sure that the container is appropriately sized and labeled.
- Adjust the amount of 10% NBF to obtain a 20:1 ratio of fixative to tissue.
- Lay the animal’s carcass in dorsal recumbency on a clean dissection board and expose the tissues of interest.
- Isolate the tissues from the animals’ carcass with the help of dissecting forceps and scissors.
- Scrap the tissues to remove fat and unnecessary connective tissue carefully. The blood should be clean; use normal saline to rinse.
Note: Do not use distilled or tap water to rinse the tissues.
- Place the dissected tissues in the container containing 10% NBF.
Postmortem Collection and Perfusion of Lung Tissue
- Fill a container with 10% NBF and adjust the volume of 10% NBF to obtain a 20:1 ratio of fixative to tissue.
- Lay the animal to be dissected in dorsal recumbency on a clean dissection board.
- Expose and display the trachea, heart, and lungs.
- Remove the skin and muscle overlying the ventral thoracic and cervical regions using the dissecting forceps and scissors.
- Trim the ribcage to expose the heart and lungs near the clavicle to open space, ample enough to observe all the lobes of the lung thoroughly.
- Remove the neck muscles surrounding the sternum and ribs and ranging to the jaw, including those overlying the trachea.
- Make two cuts by inserting the scissors under the anterior edge of the rib cage, one cut on either side, to remove the section of bone overlying the trachea.
- With the forceps hold the trachea near the jaw and cut the trachea entirely by placing the scissors above the forceps
- Pull out the trachea with the help of the forceps and cut the ventral tissue connections with scissors until all the thoracic tissues are removed from the body.
- Place the lungs flat on the shelf.
- Stitch the trachea with suturing material loosely.
- Fill a syringe with a fixative and attach a needle, small enough to enter the trachea (For mice, a 1ml or 3ml syringe with a 26 gauge needle works well. For rats, a 5ml syringe with an 18 gauge syringe is accurate).
- Hold the trachea with the forceps and insert the needle into the trachea. Fill the lungs with fixative slowly. Keep filling until the lungs are fully inflated. Do not over-or underinflate. The amount of fixative needed depends on the age, strain, and health of the animal.
- Fluid seeping and foaming from the tissues indicate over-inflation.
- Flat lungs indicate under-inflation.
- Withdraw the needle from the trachea.
- Tighten the stitches on the trachea to prevent backflow of fixative out of the lungs.
- Keep the inflated lungs into the fixative with a 20:1 fixative to tissue ratio.
Postmortem Collection of Brain
- Bring an appropriately sized, labeled container(s) and fill in the required amount of 10% NBF and adjust the amount of 10% NBF to obtain a 20:1 ratio of fixative to tissue.
- Place the animal carcass in ventral recumbency on a clean dissection board.
- Remove the skin and the muscles surrounding the calvaria with the help of dissecting scissors.
- Dislocate and remove the head from the animal’s body.
- Insert the bottom blade in the foramen magnum (the opening where the skull opens into the spinal canal) with the help of small scissors, keep the scissor tips pointed upwards, and begin cutting through the midline of the calvaria.
- Reflect both halves of the calvaria and expose the brain.
- Place the exposed brain encase in the skull into a fixative.
- Invert the skull gently so that the tissues fell from the skull because of the gravity.
- Move the forceps under the brain starting at the olfactory lobes and along the outer edge of the brain, moving under the cerebrum and towards the cerebellum carefully. Gently pinch the connective tissue or nerves, with the help of the forceps, inhibiting the brain from falling from the skull.
- Place and keep the brain into the fixative using an approximate 20:1 ratio of tissue to fixative.
Postmortem Collection of Respiratory Aspirate
- Place the animal to be dissected in dorsal recumbency on a clean dissection board.
- For bronchial aspirate collection in rats, get access to the respiratory tract through the trachea. For nasal aspirate in rats, reach the respiratory tract either through the trachea or the nasopharyngeal meatus. For bronchial or nasal aspirates in mice, approach the respiratory tract from the nasopharyngeal meatus.
- Tracheal Access (recommended for rats):
- Expose the subcutaneous tissues by moving the skin away from the cervical area.
- Separate the salivary glands and cervical musculature to expose and visualize the trachea.
- With the help of sterile dissecting instruments, cut the trachea to reach its lumen.
Note: Maintain asepsis throughout the collection.
- Nasopharyngeal Meatus Access:
- Cut the temporomandibular (jaw) joint and move the mandible away from the maxilla to expose the nasopharyngeal meatus.
Note: Maintain asepsis throughout the collection.
- Draw approximately 1ml of sampling fluid into a sterile pipette.
Note: Sampling fluid may be normal saline, phosphate-buffered saline, or trypticase soy broth.
- Bronchial Aspirate:
- Inject the sampling fluid slowly into the bronchi and the lung by caudally inserting the pipette into the trachea. Pipette out the sampling fluid from the bronchi and the lungs and remove the pipette from the trachea.
- Nasal Aspirate:
- Cranially direct and insert the pipette into the nasopharyngeal meatus (mice) or tracheal lumen (rats), and pour the sampling fluid into the nasal cavity.
- Access the nasal cavity by contacting the nasal palate with the pipette tip, or by observation of fluid forced into the cavity, seen as menisci forming at the nasal orifice (nares) or as fluid visible through the translucent oral palate.
- Draw the sampling fluid from the nasal cavity into the pipette and remove the pipette from the meatus.
- Transfer the sample to an appropriate media or container for testing.
Maintain asepsis throughout the procedure.
Dissection of Mouse Parotid Glands
- Euthanize the mice using the cervical dislocation method.
- Cut and open the skin of the chest and head/neck region from the epigastrium toward the limbs.
- Open the thorax by making a trapezoid cut.
- Make a small incision in the apex of the left ventricle, insert the perfusion cannula into the incision and gently push into the aortic arch.
- Perfuse [4% paraformaldehyde (PFA) in PBS with RNase-free water at pH 7.4] for 2–3 min.
- Lift the parotid glands with a pair of forceps to locate the borders of the organ and cut out, including a part of the main excretory duct.
- Separate the parotid glands from the surrounding tissues.
Mouse Dorsal Root Ganglia (DRG) Isolation Protocol
Spinal Column Isolation
- After submerging the fur with 70 % ethanol, make a small incision in the dorsal skin at the level of the hips, and remove the pelt from the head to hind limbs.
- Remove the head by cutting at the base of the skull (C1–2 level) and cut the arms beneath the shoulders to aid the removal of the skin.
- Make an incision through the abdominal wall muscles and continue laterally to the spinal column in both directions.
- Before the viscera detachment, incise the ribs closer to the spinal column from both sides.
- Cut the femurs, and remove the spinal column by cutting transversely at the level of the femurs.
Spinal Cord Exposure
- Cut the muscles, fat, and other soft tissues from the spinal column with the help of curved scissors. The T13 level DRG pair present at the caudal ribs is used as a landmark.
- Remove the spinal nerves projected from the column.
- Once cleared of soft tissues, cut the column into three pieces, with one cut at the level of the last rib to orientate the dissection.
- Place the column segments dorsally with the side facing up.
- Use thick forceps to secure the spinal column dorsal side up, before cutting it into two halves along the midline.
- Pin the Hemi-segments of the spinal column in Petri dishes with the medial side up, using two insect pins through intervertebral discs, before rinsing with ice-cold PBS.
DRG Extraction and Cleaning
- Peel the spinal cord from the pinned column in a rostral to caudal direction.
- Identify the transparent meningioma sheets of tissue covering the DRG, and carefully remove it, making the DRG easier to see.
- Dissect out the individual ganglia by grasping and lifting with forceps, and find the distally projecting axon bundles on the lateral side of the DRG. Do not damage the DRG with the forceps.
- Pinout the DRG via their axons, and remove any residual meninges, before cutting the axons close to the DRG.
Applications
Diagnostic Necropsy and Selected Tissue and Sample Collection in Rats and Mice (Parkinson et al., 2011)
Proper collection of tissues for histological processing may impact the quality of research. As proper inflation of the tissues with fixative is necessary, the lung collection and perfusion are challenging to enable thorough histological evaluation. Brain collection can be similarly challenging as the tissue is soft and can be easily damaged. Collection of the mesenteric lymph node enables the detection of many infectious agents as the enteric viruses persist in the lymph node for longer. Infectious agents in the respiratory tract can be identified by performing bacterial culture or PCR testing of nasal and bronchial fluid aspirates taken at necropsy. The dissection procedure helps the researchers to perform histological analysis as well as anatomical investigations on rodents.
Rapid Isolation of Mouse Dorsal Root Ganglia (DRG) (Sleigh, Weir, & Schiavo, 2016)
The procedure involves the dissection of the spinal column, by cutting from the base of the skull to the level of the femurs, before extracting the DRG and removing unwanted axons. The protocol allows the easy and rapid isolation of DRG with minimal practice and dissection experience. The method is faster and simpler than
in situ column extraction of the ganglia after dorsal laminectomy. Also, the approach is less time-consuming, efficient, and safe for the collection of DRG. The method increases the chances of collecting healthy primary DRG cultures with high-quality and reproducible experiments using the DRG tissue.
Isolation of High-Quality RNA from Murine and Human Parotid Tissue (Watermann et al., 2016)
The procedure is a simple and optimized surgical method to perfuse and isolate murine parotid glands. The research compared the two common RNA extraction methods for their high-quality yields containing intact RNA from human and murine parotid gland tissues either snap-frozen or immersed in RNAlater stabilization solution. The murine and human parotid tissues exhibited the best RNA quality showing a significant difference between the perfusion-fixed group and the other experimental groups, independent of the isolation method.
Precautions
- Keep a note of the preservative solution.
- Rinse the preserved animals under running water immediately upon removal from the preservative solution.
- Work in a well-ventilated area.
- It is recommended that contact lenses should not be worn while dissecting animals that are in preservative solution. As the fumes from the solution can penetrate between the eye and contact lens are irritating the eyes. Wear prescription glasses instead, with safety glasses over them.
- Be aware of issues such as allergies and chemical sensitivities from handling freshly euthanized or recently defrosted or preserved animals.
- Be aware of possible microbial aerosols and unpleasant odors released from freshly euthanized or recently defrosted animals if the stomach/intestines are accidentally cut.
- If using frozen animals, defrost overnight in a refrigerator beforehand and dissect within 24 hours.
- Observe good hygiene practices throughout the procedures by keeping the hands away from the mouth, nose, eyes, and face during and after the dissection and wash the hands immediately after handling the dissection material.
Summary
- In biological research, dissection is the process of cutting and disassembling the body parts of the laboratory animals to study their anatomical structures.
- Rodents are the most preferred species to be used as animal models for biomedical research due to their anatomical, physiological, and genetic similarities to humans.
- The rodents are euthanized before the dissection procedures. Different methods of euthanasia are applied, enabling a rapid death with the reduced pain for the animal and the safety of the field workers should be selected.
- The dissection procedure helps the researchers to perform histological analysis as well as anatomical investigations on rodents.
References
- Bröllochs, A. (2017, August 11). Dissection of mouse EDL and Soleus muscles. Retrieved from Protocols.io: https://www.protocols.io/view/dissection-of-mouse-edl-and-soleus-muscles-jcrciv6?step=13
- Parkinson, C. M., O'Brien, A., Albers, T. M., Simon, M. A., Clifford, C. B., & Pritchett-Corning, K. R. (2011). Diagnostic Necropsy and Selected Tissue and Sample Collection in Rats and Mice. J Vis Exp, 54, 2966.
- Sleigh, J. N., Weir, G. A., & Schiavo, G. (2016). A simple, step-by-step dissection protocol for the rapid isolation of mouse dorsal root ganglia. BMC Research Notes, 9(82).
- Watermann, C., Valerius, K. P., Wagner, S., Wittekindt, C., Klussmann, J. P., Vogt, E. B., & Karnati, S. (2016). Step-by-step protocol to perfuse and dissect the mouse parotid gland and isolation of high-quality RNA from murine and human parotid tissue. BioTechniques, 60, 200-203.